
New York, NY – October 12, 2023 – In a challenge brought by T-Mobile US, Inc., the National Advertising Division (NAD) of BBB National Programs determined that Comcast Cable Communications, LLC provided a reasonable basis for its “Next Generation” claim for its “Xfinity 10G Network,” as well as the implied claim that it has already achieved a major technological revolution.
However, NAD recommended that Comcast:
- Discontinue its “10G” claims or qualify them to (a) make clear that Comcast is implementing improvements that will enable it to achieve “10G” and that 10G is aspirational, or (b) use “10G” in a manner that is not false or misleading, and
- Modify its advertising to avoid conveying the unqualified message that Xfinity customers’ home internet is uninterrupted during power outages.
In February 2023, Comcast rebranded its fixed internet network as “Xfinity 10G Network” to signify technological upgrades to its network that are continuing to be implemented. T-Mobile challenged the name “Xfinity 10G Network” and the claim “10G,” which appeared in a myriad of advertisements on television and online.
“10G Claims”
NAD concluded that “10G” as used in the name “Xfinity 10G Network” and “Xfinity 10G” is an express claim that means 10 Gbps or 10th Generation.
In evaluating support for this claim, NAD found that Comcast’s description of its entire network as “10G” conveys the message that all consumers on the network will receive a significant increase in speed up to 10 Gbps speeds. However, only one of Xfinity’s many plans (Gigabit Pro) can reach 10 Gbps, and to access that service tier requires the installation of fiber to the premises. Further, NAD determined that the evidence in the record was insufficient to support the broad, unqualified message that the “Xfinity 10G Network” is vastly superior to 5G.
For these reasons, NAD concluded that Comcast did not provide a reasonable basis for its 10G claims and recommended that Comcast discontinue the claims:
- “10G”
- “Xfinity 10G”
- “Xfinity 10G Network”
Alternatively, NAD stated that Comcast may modify its advertising to (a) make clear that it is implementing improvements that will enable it to achieve “10G” and that it is aspirational or (b) use “10G” in a manner that is not false or misleading, consistent with this decision. NAD noted that nothing in its decision would prevent Comcast from making a more qualified claim, if supported, about the superiority of its network over 5G.
“Next Generation” and Technological Revolution Claims
Based on upgrades to Comcast’s network reliability, lower latency, and other features, NAD determined that Comcast provided a reasonable basis for its “Next Generation” claim and implied claims that it already achieved a major technological revolution.
Power Outage Implied Claim
T-Mobile argued that Comcast’s “What a Time to Be Alive” commercials convey the message that Xfinity customers’ home internet is uninterrupted during power outages, but the claim is premature because the Xfinity website stated that “storm-ready” WiFi is “coming soon.”
As the record did not include details on the effectiveness of this monitoring technology in ensuring that customers can still stream a movie uninterrupted when power runs out, NAD found that Comcast did not provide a reasonable basis for this claim. Therefore, NAD recommended that Comcast modify its advertising to avoid conveying the unqualified message that Xfinity customers’ home internet is uninterrupted during power outages.
In its advertiser statement, Comcast stated that it will appeal NAD’s decision because it “disagrees with NAD’s decision, including NAD’s determination that the “Xfinity 10G Network” brand name constitutes an ‘express claim’” and NAD’s assessment of survey evidence submitted by T-Mobile.
T-Mobile will cross-appeal NAD’s determination regarding Comcast’s “Next Generation” and technological revolution claims.
Appeals of NAD decisions are made to the BBB National Programs’ National Advertising Review Board (NARB), the appellate-level truth-in-advertising body of BBB National Programs.
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. This press release shall not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and leveling the playing field for business.
Contact Information
Name: Jennie Rosenberg
Email: jrosenberg@bbbnp.org
Job Title: Media Relations
Comcast Appeals National Advertising Division Recommendation to Discontinue or Modify Xfinity “10G” Claim

New York, NY – October 12, 2023 – In a challenge brought by Verizon Communications Inc, the National Advertising Division (NAD) of BBB National Programs recommended that Comcast Cable Communications, LLC discontinue the claim “10G” for its Xfinity 10G Network or qualify the claim to make clear that Comcast is implementing improvements that will enable it to achieve 10G and that 10G is aspirational or use “10G” in a manner that is not false or misleading.
In February 2023, Comcast rebranded its fixed internet network as “Xfinity 10G Network” to signify technological upgrades that continue to be implemented. Verizon challenged the name “Xfinity 10G Network” and the claim “10G,” which appeared in a myriad of advertisements online and on television.
NAD concluded that “10G” as used in the name “Xfinity 10G Network” and “Xfinity 10G” is an express claim.
In evaluating this claim, NAD found that Comcast’s description of its entire network as “10G” conveys the message that all consumers on the network will receive a significant increase in speed up to 10 Gbps. However, NAD determined that the 10 Gbps message was not substantiated because only one of Xfinity’s many plans (Gigabit Pro) can reach 10 Gbps, and access to that service tier requires installation of fiber to the premises. Further, NAD concluded, that to the extent that consumers understand 10G as referring to 10th generation mobile technology, this message is also unsupported.
For these reasons, NAD recommended that Comcast discontinue the claim “10G.”
Alternatively, NAD stated that Comcast may modify the claim by (a) qualifying it to make clear that they are implementing improvements that will enable it to achieve 10G and that 10G is the aspiration, or (b) using it in a manner that is not false or misleading consistent with this decision.
In its advertiser statement, Comcast stated that it will appeal NAD’s decision because it “disagrees with NAD’s decision, including NAD’s determination that the Xfinity 10G Network brand name constitutes an ‘express claim.’”
Appeals of NAD decisions are made to the BBB National Programs’ National Advertising Review Board (NARB), the appellate-level truth-in-advertising body of BBB National Programs.
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. This press release shall not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and leveling the playing field for business.
Contact Information
Name: Jennie Rosenberg
jrosenberg@bbbnp.org
Job Title: Media Relations
National Advertising Division Recommends Louisiana-Pacific Modify or Discontinue Certain Home Siding Claims; Finds Other Claim Supported

New York, NY – October 12, 2023 – In a challenge brought by James Hardie Building Products, Inc., the National Advertising Division (NAD) of BBB National Programs determined that Louisiana-Pacific Corporation (LP) provided a reasonable basis for the claim that “LP®BuilderSeries®Lap Siding is lighter than fiber cement, allowing you to carry more boards with less effort.” However, NAD recommended certain quantified and unquantified superiority claims about its SmartSide, SmartSide ExpertFinish, and BuilderSeries engineered wood products compared to fiber cement products be modified or discontinued.
Siding products come in various materials including fiber cement, wood, engineered wood, and vinyl with differing price and performance attributes such as protection, durability, and aesthetics.
As support for LP’s superiority claims, which appeared on its website and in videos showing timed installations of company-engineered wood products versus fiber cement products, LP relied on the results of time-motion studies conducted by a leading construction cost data provider, commissioned by LP.
Faster Installation Claims
James Hardie challenged certain quantified installation claims made by LP that touted a precise comparative installation advantage of LP-engineered wood products over fiber cement products including, for example, that “Overall, LP®SmartSide®products installed 30% faster than fiber cement.”
NAD determined that the studies did not reliably support such precise quantified results due to certain non-product variables that may have impacted the results and recommended that the quantified installation claims be discontinued. Further, NAD recommended that LP discontinue the videos or modify them to avoid conveying any quantified installation advantage claims.
NAD found, however, that the studies reasonably support a qualified comparative message that, in the context of the study, LP’s SmartSide and SmartSide ExpertFinish engineered wood products install faster than fiber cement. Accordingly, NAD recommended that LP:
- Modify the unquantified claims that “LP SmartSide Primed and LP SmartSide ExpertFinish products both installed significantly faster than fiber cement” and “LP SmartSide engineered wood siding…is much easier—and faster—to install” to clearly and conspicuously disclose the limited testing on which such comparative installation claims are based to avoid conveying a message that the engineered wood products will install faster than fiber cement products under all conditions, and
- Remove the word “significantly” from the challenged claim.
Weight Claims
NAD recommended that LP modify the claim “LP®SmartSide®Lap weighs 45% less than fiber cement” to clearly and conspicuously disclose the basis of the weight comparison (i.e., that the comparison is based on the weight of equal 1’ pieces of the products). NAD found that this modification would avoid conveying an unsupported message that LP’s claim is based on a comparison of the products prior to being cut.
NAD also found a reasonable basis of support for the challenged unquantified claim that “LP®BuilderSeries®Lap Siding is lighter than fiber cement, allowing you to carry more boards with less effort.”
Waste Claim
Because NAD determined that the studies’ small sample size does not provide a reliable measure of quantified comparative performance, NAD recommended that LP discontinue the claim that “Primed LP®SmartSide®Lap reduced waste by 7% compared to fiber cement.”
Implied Claims
NAD recommended that LP modify its advertising to avoid conveying the unsupported message that by using its engineered wood products instead of James Hardie fiber cement products, consumers can spend less on labor costs. However, NAD noted that nothing in its decision prevents LP from making a more limited comparative savings claim for which it has adequate support.
In its advertiser statement, LP agreed to “modify/discontinue the advertising statements addressed in NAD’s recommendation” even though it “respectfully disagrees with NAD’s decision in several respects, especially with regard to whether the third-party studies adequately supported the precise quantified results.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. This press release shall not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and leveling the playing field for business.
Contact Information
Name: Jennie Rosenberg
Email: jrosenberg@bbbnp.org
Job Title: Media Relations
National Advertising Review Board Recommends Google Discontinue Claim that YouTube TV Service is “$600 Less Than Cable”

New York, NY – October 11, 2023 – A panel of the National Advertising Review Board (NARB), the appellate advertising body of BBB National Programs, recommended that Google, LLC discontinue the claim that its YouTube TV service is “$600 less than cable.”
The advertising at issue had been challenged by Charter Communications, Inc. in a National Advertising Division’s (NAD) Fast-Track SWIFT challenge, an expedited process designed for single-issue advertising cases. The claim appeared in two of Google’s commercials for YouTube TV service. Following NAD’s decision (Case No. 7233), Google appealed NAD’s recommendation to discontinue the challenged advertising claim.
The challenged “$600 less than cable” claim, was accompanied by a disclosure identifying “comparable standalone cable” as the basis of comparison. The price calculation underlying the challenged claim included the cost of two set-top boxes per household for “standalone cable” services.
The NARB panel determined that the commercial disclosures were not clear and conspicuous.
Further, in agreement with NAD, the NARB panel concluded that at least one reasonable interpretation of the challenged claim is that YouTube TV is $600 less than any comparable service available from companies traditionally associated with cable services. However, this comparison does not align with the challenged claim because:
- Many households can subscribe to basic Spectrum service without renting cable boxes, therefore Google failed to justify the cost of two set-top boxes in its price comparison, and
- In certain markets, cable providers offer regional sports networks (RSNs) but YouTube does not, therefore Google did not have a valid reason for adding the cost of Spectrum’s Sports View option to the price comparison.
For these reasons, the NARB panel adopted NAD’s recommendation that Google discontinue the claim that its YouTube TV service is “$600 less than cable.”
Google stated that it “disagrees with NARB’s determination that people watching the challenged commercials will somehow understand ‘cable’ to mean something other than traditional cable television,” however it “intends to modify or cease the disputed advertising claim.” Google further stated that, at a later date it “may reconsider the claim based on updated information.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Review Board (NARB): The National Advertising Review Board (NARB) is the appellate body for BBB National Programs’ advertising self-regulatory programs. NARB’s panel members include 85 distinguished volunteer professionals from the national advertising industry, agencies, and public members, such as academics and former members of the public sector. NARB serves as a layer of independent industry peer review that helps engender trust and compliance in NAD, CARU, and DSSRC matters.
Contact Information
Name: Jennie Rosenberg
jrosenberg@bbbnp.org
Job Title: Media Relations
Privacy Watchdog Brings Etsy into Compliance with Digital Advertising Privacy Best Practices

McLean, VA – October 10, 2023 – BBB National Programs’ data privacy watchdog, the Digital Advertising Accountability Program (DAAP), has brought Etsy Inc.’s website and mobile application into compliance with the Digital Advertising Alliance’s (DAA) Self-Regulatory Principles for online interest-based advertising.
Etsy is a New York-based e-commerce company known for its online marketplace of vintage and handmade goods, operating through etsy.com and mobile apps. DAAP also reviewed Etsy’s online platforms in 2015.
As part of its ongoing monitoring activity and to ensure ongoing compliance, DAAP revisits companies and their products previously reviewed.
While examining Etsy’s website as well as its mobile application, DAAP identified issues with transparency, enhanced notice, and consumer access to exercise control over data collection for interest-based advertising (IBA). In addition, in the Etsy mobile application, DAAP identified issues violating the first-party cross-app provisions of the DAA Principles Mobile Guidance.
In response to DAAP’s inquiry, Etsy conducted a comprehensive review of its compliance with the DAA Principles to identify areas in its compliance protocols that needed strengthening. After consulting with DAAP on its plan to come into compliance, Etsy committed to completing the following actions:
- Changing the “interest-based ads” website footer link to redirect users to a specific section of the Cookie Policy titled “Interest-based Ads & Marketing Services.”
- Significantly editing the IBA section to bring all elements of DAA enhanced notice together, including a description of Etsy’s third-party IBA practices, a link to, and description of, industry-developed IBA opt-out tools, and a statement of adherence to the DAA Principles.
- Adding language at the top of its privacy policy that reads “Learn more about interest-based advertising and your choices, here” with a hyperlink that takes users directly to the “Interest-based Ads & Marketing Services” section of the privacy policy to ensure users could easily access this information from the application store pages in the app store.
In its statement, Etsy stated “At Etsy, we care deeply about privacy and strive to be transparent about our practices with our community of buyers and sellers. We appreciate the opportunity to participate in the DAA’s Accountability Program and are pleased with the Program’s recognition that Etsy is compliant with its self-regulatory requirements.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of DAAP decisions, visit the DAAP Decisions and Guidance webpage.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the Digital Advertising Accountability Program: The Digital Advertising Accountability Program (DAAP), a division of BBB National Programs, was developed by the Digital Advertising Alliance (DAA) to enforce industry self-regulation principles for data privacy in online and mobile advertising, holding companies accountable to the DAA’s Privacy Principles. DAAP provides guidance to companies looking to comply with industry principles and responds to complaints filed by consumers about online privacy.
Contact Information
Name: Jennie Rosenberg
jrosenberg@bbbnp.org
Job Title: Media Relations
Momentum grows in 2nd year of MICHELIN Guide Vancouver

- Okeya Kyujiro earns a MICHELIN Star, bringing city’s total to nine
- Five more Bib Gourmand, plus four special awards, also revealed
- 77 restaurants constitute 2023 selection – up from 60 in 2022
- 2023 edition also includes 21 different types of cuisine
VANCOUVER, British Columbia, Oct. 5, 2023 — A new MICHELIN-Starred restaurant, five new Bib Gourmand and 12 new Recommended restaurants crossed the stage Thursday night at the Fairmont Pacific Rim to highlight the announcement of the 2023 MICHELIN Guide Vancouver.
The MICHELIN Guide also revealed four special awards at the ceremony. Overall, the MICHELIN Guide inspectors awarded Stars to nine restaurants – eight of which earned Stars in 2022. The full 2023 selection comprises 77 restaurants and 21 types of cuisine.
“The famously anonymous MICHELIN Guide inspectors once again were impressed with the culinary community here,” said Gwendal Poullennec, the International Director of the MICHELIN Guides. “This sort of steady growth is what we often see in second-year selections, and it is definitely a harbinger of great things to come. We are very proud of the passionate chefs and restaurant teams here in Vancouver, and they make their city very proud.”
Okeya Kyujiro, helmed by Chef Takuya Matsuda, earned one MICHELIN Star for the first time. Here are the inspector notes (inspectors’ comments in full on the MICHELIN Guide website and mobile app):
Okeya Kyujiro (Japanese cuisine)
Hosts dressed in stunning traditional clothing guide you to a dark room, lit only by the faintest glow from votive candles. A black curtain is raised ceremoniously only when the clock strikes the precise minute of your seating. These are the first clues that this is far from your typical omakase. From the premium, hyper-seasonal fish to the demonstration of sasagiri (traditional Japanese bamboo leaf carving), it is a memorable show from start to finish. Highlights include chawanmushi with cherry blossom, shatteringly crispy tempura sandbar fish, spicy firefly squid on a bamboo skewer and a presentation of two uni petals from different Japanese waters served with seaweed jam.
Bib Gourmand
The MICHELIN Guide inspectors added five restaurants to the Bib Gourmand list, which recognizes eateries for great food at a great value: Farmer’s Apprentice, Karma Indian Bistro, Motonobu Udon, Seaport City Seafood and Sushi Hil.
Special Awards
In addition to the Bib Gourmands and Stars, the Guide announced four special awards:
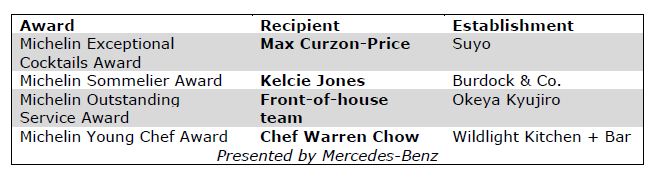
The MICHELIN Guide Ceremony is presented with the support of Capital One. Transportation provided by Mercedes-Benz.
Hotel
The restaurants join the MICHELIN Guide selection of hotels, which features the most unique and exciting places to stay in Vancouver and throughout the world.
Each hotel in the selection has been chosen by MICHELIN Guide experts for its extraordinary style, service, and personality — with options for all budgets — and each can be booked directly through the MICHELIN Guide website and app. The selection for Vancouver features the city’s most spectacular hotels, including design-forward luxury boutiques like the Douglas, standouts from our “Plus” collection like the Loden and the Opus, old-world elegance like the Wedgewood, and dependable luxury stalwarts like the Fairmont Pacific Rim.
The MICHELIN Guide is a benchmark in gastronomy. Now it’s setting a new standard for hotels. Visit the MICHELIN Guide website, or download the free app for iOS and Android, to discover every restaurant in the selection and book an unforgettable hotel.

The 2023 MICHELIN Guide Vancouver selection:

Vancouver’s 2023 MICHELIN-Starred restaurants
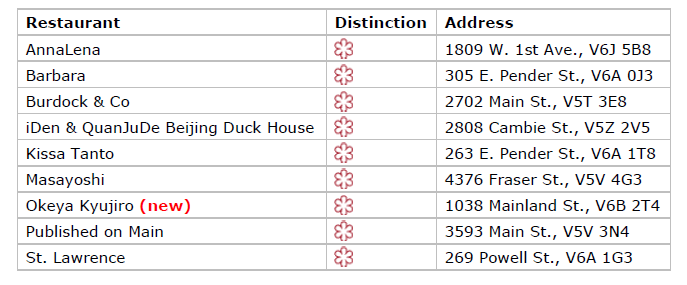 Vancouver’s 2023 Bib Gourmand restaurants
Vancouver’s 2023 Bib Gourmand restaurants
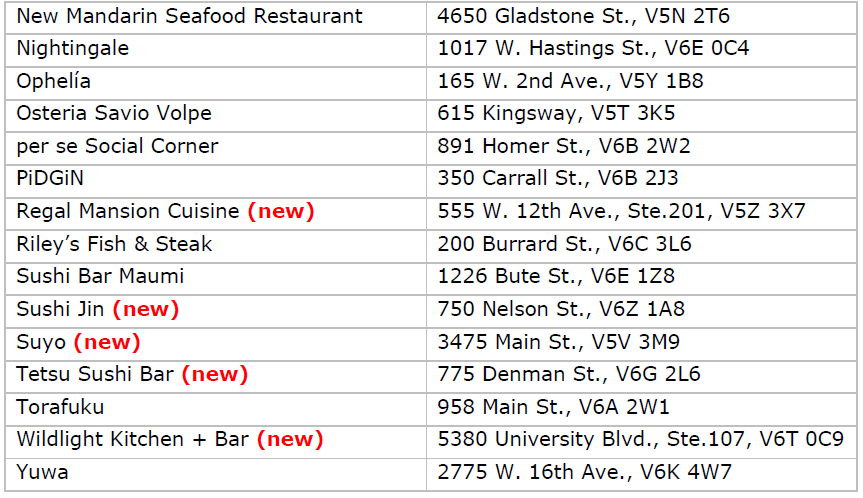
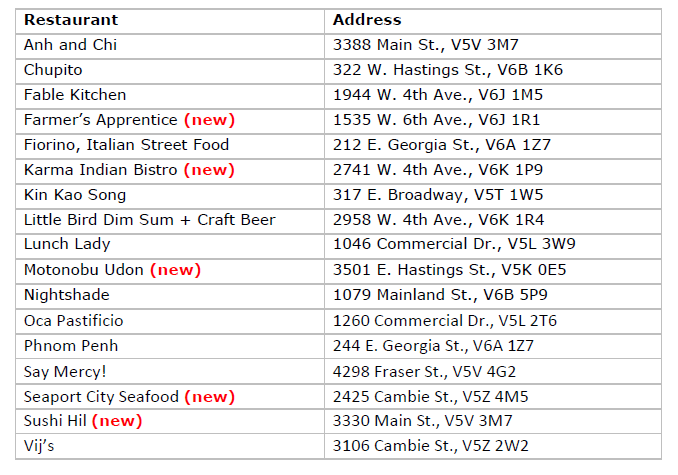 Vancouver’s 2023 Recommended restaurants
Vancouver’s 2023 Recommended restaurants
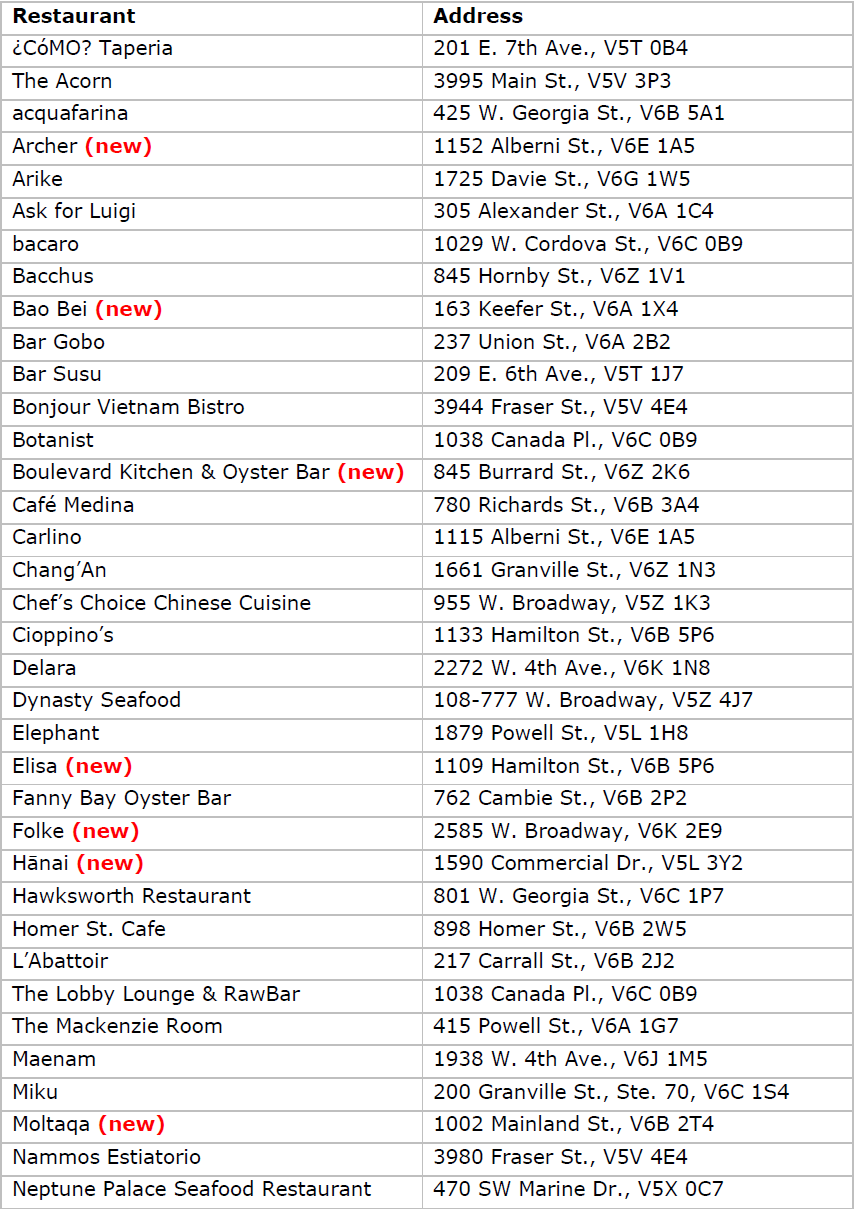
About Michelin North America, Inc.
Michelin, the leading mobility company, is working with tires, around tires and beyond tires to enable Motion for Life. Dedicated to enhancing its clients’ mobility and sustainability, Michelin designs and distributes the most suitable tires, services and solutions for its customers’ needs. Michelin provides digital services, maps and guides to help enrich travel and make them unique experiences. Bringing its expertise to new markets, the company is investing in high-technology materials, 3D printing and hydrogen, to serve a wide variety of industries — from aerospace to biotech. Headquartered in Greenville, South Carolina, Michelin North America has approximately 23,000 employees and operates 34 production facilities in the United States and Canada. (michelinman.com)
About Capital One
At Capital One we’re on a mission for our customers – bringing them best-in-class products, rewards, service, and experiences. Capital One is a diversified bank that offers products and services to individuals, small businesses and commercial clients. We use technology, innovation, and interaction to provide consumers with products and services to meet their needs. Through Capital One Dining and Capital One Entertainment, we provide our rewards cardholders with access to unforgettable experiences in the areas they’re passionate about, including dining, music and sports. Learn more at capitalone.com/dining and capitalone.com/entertainment.
For more information, contact:
Andrew Festa
Michelin North America andrew.festa@michelin.com
Devon Gunn Capital One
devon.gunn@capitalone.com Phone: 571-308-4762
Contact Information
Name: Andrew Festa
Email: andrew.festa@michelin.com
Job Title: Press Officer
Mint Mobile Appeals National Advertising Division Recommendation to Discontinue or Modify Claim that its Unlimited Plan is “Now Just $15/Mo.”

New York, NY – October 4, 2023 – In a Fast-Track SWIFT challenge brought by AT&T Services, Inc., the National Advertising Division (NAD) of BBB National Programs recommended that Mint Mobile, LLC discontinue or modify the claim that its Unlimited Plan is “now just $15/mo.”
Mint Mobile offers prepaid phone plans, which, unlike plans offered by AT&T and other major wireless carriers, require customers to pay upfront before receiving service. Mint Mobile’s $15/mo price for its Unlimited plan is a promotional rate that is only in effect for three months. After three months of service, the monthly rate increases with the exact amount of the increase dependent on which plan the consumer selects.
Fast-Track SWIFT is an expedited process designed for single-issue advertising cases brought to NAD. At issue for NAD was whether the nature of Mint Mobile’s promotional offer was adequately disclosed.
In banner ads, on social media, and in a television commercial, Mint Mobile advertises that the price of its Unlimited Plan is “now just $15/mo.” However, NAD found that the challenged advertising does not adequately disclose that the $15 monthly service is a promotional offer for only three months of service.
Accordingly, NAD recommended that Mint Mobile discontinue the claim that its Unlimited Plan is “now just $15/mo” or clearly and conspicuously disclose that the offer is a promotional offer for three months of service as part of the main claim or in similar size text and font in close proximity to the main claim.
In its advertiser statement, Mint Mobile stated that it will appeal NAD’s decision because it “respectfully disagrees with NAD’s determination that the contours of the promotion were inadequately disclosed and that consumers reasonably interpret the phrase, ‘now just $15/mo’ to mean that ‘the rate is one in perpetuity, or of longer duration similar to introductory pricing offered by other wireless carriers’” because Mint Mobile only offers “wireless service plans in three-, six-, and twelve-month increments.”
Appeals of NAD decisions are made to the BBB National Programs’ National Advertising Review Board (NARB), the appellate-level truth-in-advertising body of BBB National Programs.
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. This press release shall not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and leveling the playing field for business.
Contact:
Jennie Rosenberg
Media Relations
BBB National Programs
press@bbbnp.org
STATE REPRESENTATIVES HARRIS AND SCHWEYER KICK-OFF PENNSYLVANIA EDUCATION TOUR
Pennsylvania Education Tour Kicks-Off in Pittsburgh October 5
HARRISBURG, PA., (October 3, 2023)– House Appropriations Committee Chairman Jordan Harris and House Education Chairman Pete Schweyer will kick-off the Pennsylvania Education Tour in Pittsburgh on Thursday, October 5, 2023.
As part of a joint effort between the House Appropriations and Education Committees, the statewide tour will examine education funding broadly in the wake of the Commonwealth Court’s ruling declaring Pennsylvania’s public education funding system unconstitutional and to assess efforts to address educational inequality in Pennsylvania, including existing and additional options to education.
“Now is not the time to be adversarial on the issue of education; it is the time to be visionary, it is the time to be inspirational and aspirational about what we could do as a Commonwealth with regards to our children,” said Representative Jordan Harris, Majority Chairman of the House Appropriations Committee. “It is incumbent upon us to make the necessary changes to ensure that every child in Pennsylvania, regardless of zip code or economic status, has the opportunity to propel themselves, their family, and their community, upward through the power of education.”
The Pennsylvania Education Funding Tour will address the shared concerns beyond educational funding related to staffing shortage issues, school safety, infrastructure investments, and access to high-quality and equitable
schools. Tour stops throughout the state will invite parents, residents, community leaders, and education experts to come together, provide testimony, and collectively take on the responsibility of supporting and realizing equitable and safe schools for all Pennsylvania students.
“The challenge is more than just a financial one,” said Representative Pete Schweyer, Majority Chairman of the House Education Committee. “We must re-examine and reimagine the education system in Pennsylvania- not just how many dollars we spend on education but also how those dollars are being spent. That is why we, the Education and House Appropriations Committee members, will be embarking on this statewide tour throughout the Commonwealth to learn from experts on issues including funding challenges, school facilities, school-based mental health, and academic curriculum.”
The full list of Pennsylvania Education Tour dates and locations are as follows:
- October 5- Allegheny Traditional Elementary Academy, 810 Arch Street, Pittsburgh, PA 15212
1 p.m. – 4:30 p.m. Public Hearing & 6:30 p.m. – 7:30 p.m. Public Comment
- October 6- Community College of Beaver County, Athletic & Events Center, 1 Campus Dr., Monaca, PA 15061
10 a.m. – 1 p.m. Public Hearing
- October 24- School District of Philadelphia, Administrative Building, Suite 301 440 N. Broad, Philadelphia, PA 19130
1 p.m. – 4 p.m. Public Hearing & 6 p.m. – 7:30 p.m. Public Comment
- October 25- Community College of Philadelphia Library & Learning Commons, Exhibit Hall 1700 Spring Garden Street, Philadelphia, PA 19130
10 a.m. – 1 p.m. Public Hearing
- November 2- Kings College, McGowan School of Business, Burke Auditorium 131 N. River St., Wilkes-Barre, PA 18711
2 p.m. – 5 p.m. Public Hearing
- November 16 – Norristown, PA
1 p.m. – 4 p.m. Public Hearing
- November 17- Penn Wood High School Auditorium, 100 Green Ave., Lansdowne, PA 19050
10 a.m. – 1 p.m. Public Hearing
For more on the Education Funding Tour, visit https://www.houseappropriations.com/EducationTour and follow the tour on YouTube, Facebook, and X (formerly known as Twitter).
Contact Information
Name: Christina Fonseca
Email: cfonseca@hacd.net
Job Title: Director of Communications
The post-pandemic legal bubble is under pressure – and it’s showing. Latest LexisNexis research predicts slowing growth for 2024 legal market.
Market challenges might finally be hitting the seemingly resilient legal sector. The latest GLP Index predicts growth will drop to only +2% in 2024 from 2023’s +6% growth.
LONDON, 3 October 2023 – Today, LexisNexis Legal & Professional®, a leading global provider of legal information and analytics, has released the latest “Gross Legal Product (GLP) Index”. This report predicts that the legal sector will grow by +2% in 2024 – suggesting that its growth trajectory is slowing.
Despite exceptionally challenging market conditions over the past three years, the Index revealed that legal demand grew by +22% in 2021, +3% in 2022 and is expected to grow by +6% in 2023.
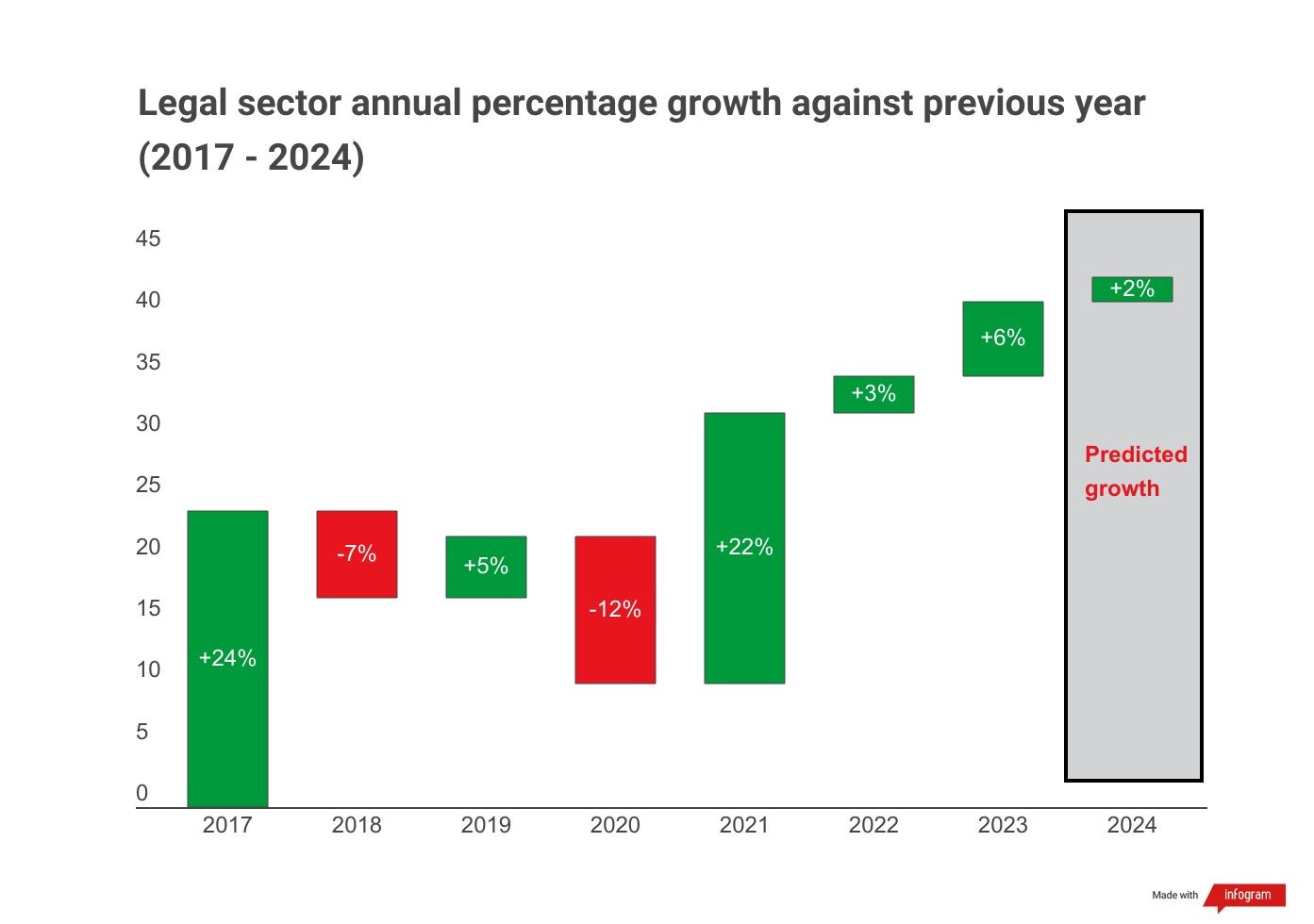
However, the latest edition of the GLP Index, which pulls historic data from hundreds of sources to predict future demand across the legal market, reveals market challenges may finally be catching up with the legal sector. These findings align with other recent events in the legal market, such as several high-profile law firms considering redundancies.
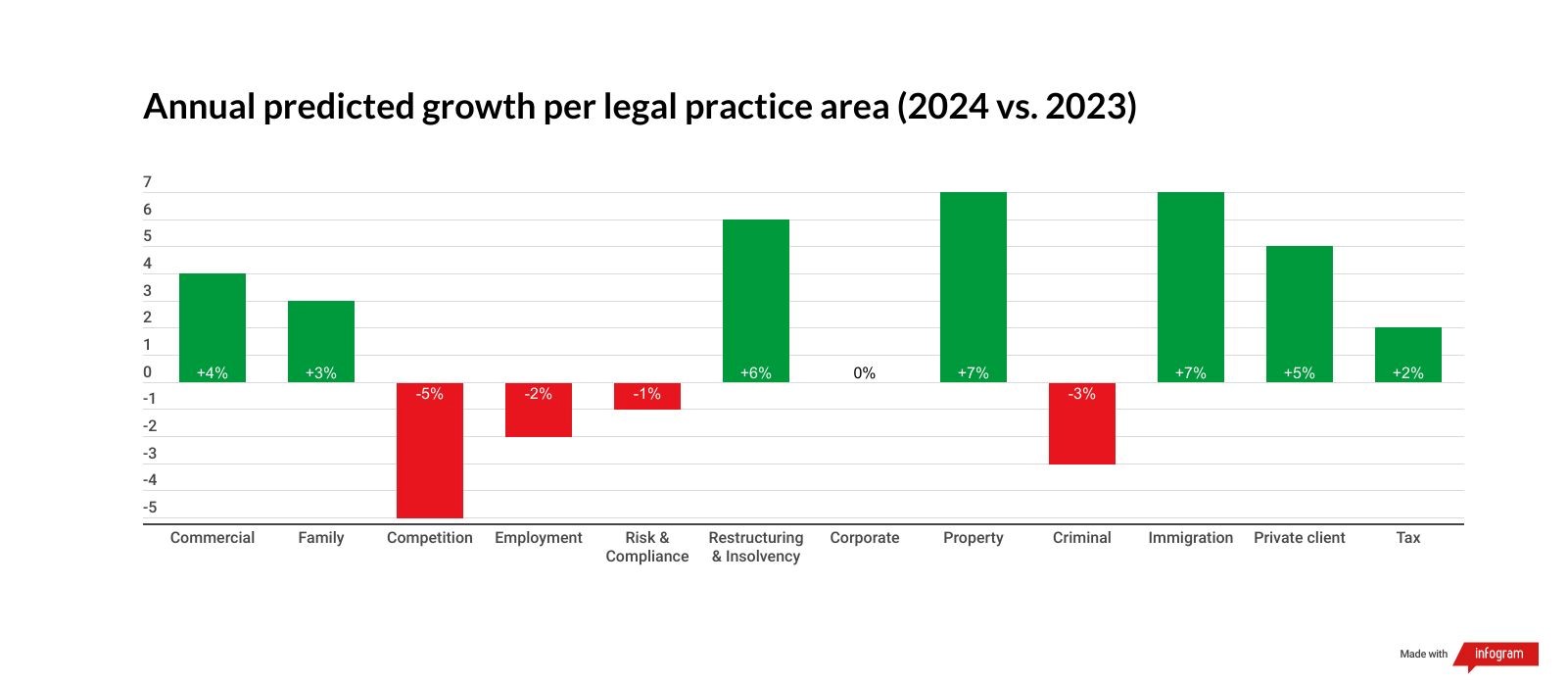
When looking at specific areas of the law, the Index predicts strong growth across property (+7%), immigration (+7%) and restructuring and insolvency law (+6%). Both property and immigration law rebounded in the previous GLP Index after several years of decline. The former has benefited from a record number of newly-completed dwellings (a 9% increase), while the latter has seen the total number of work visas issued almost double, with student visas, settlement applications and citizenship applications also on the rise.
Demand for competition (-5%) and risk and compliance law (+1%), on the other hand, will flatten next year after experiencing steady growth throughout 2022 and 2023. Practice areas that are challenged include criminal law, which is predicted to see negative growth (-3%) for the third year running, and employment law (-2%).
The report’s editor, Dylan Brown, says:
“Considering the geopolitical and macroeconomic headwinds that have battered the professional services industry for the last 18 months, subdued growth is an overwhelmingly positive message to be sharing. Recent growth speaks more to the legal sector’s resilience, ability to innovate when needed, and drive to power forward than it does to its impregnability.”
“Margins are becoming tighter” Brown continues. “So, there is also a very real risk of lawyer burn out as targets increase and the partner track presents greater risk. Firms need to continue investing in infrastructures that support their people rather than work against them.”
Notes for editors
- Read the report here: https://www.lexisnexis.co.uk/research-and-reports/glp-index-2024.html
- The attached graphs show Year on Year percentage growth across the legal sector and an overview of performance across all practice areas included in the report. They have been extracted from the report.
- Spokespeople or quotes can be provided on request.
About LexisNexis Legal & Professional
LexisNexis Legal & Professional® provides legal, regulatory, and business information and analytics that help customers increase their productivity, improve decision-making, achieve better outcomes, and advance the rule of law around the world. As a digital pioneer, the company was the first to bring legal and business information online with its Lexis® and Nexis® services. LexisNexis Legal & Professional, which serves customers in more than 150 countries with 11,300 employees worldwide, is part of RELX, a global provider of information-based analytics and decision tools for professional and business customers.
Contact Information
Matthew Leopold
Head of Brand and PR
07788435569
Matthew.Leopold@lexisnexis.co.uk
2023 MICHELIN Guide Toronto increases in Star power

- Two restaurants earn a MICHELIN Star for the first time
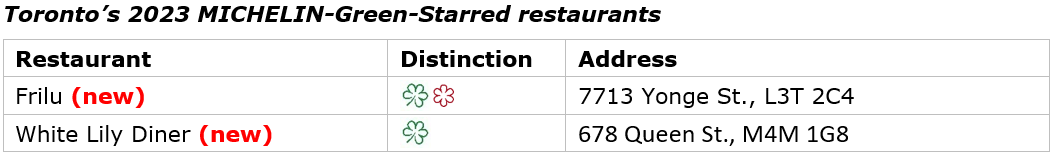
- Toronto receives its first-ever MICHELIN Green Star
- 21 Bib Gourmand, plus four special awards, also unveiled
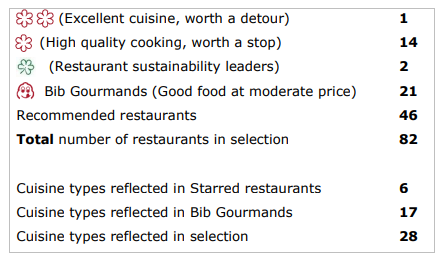
- 82 total restaurants, 28 cuisine types reflected in selection
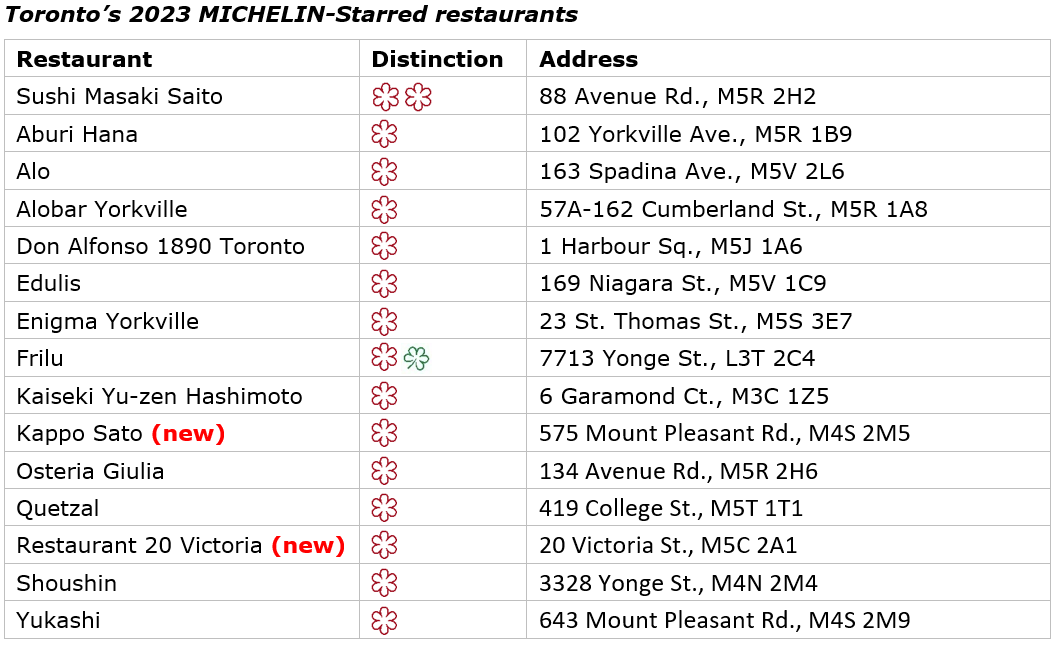
TORONTO, Sept. 27, 2023 — Two restaurants, Kappo Sato and Restaurant 20 Victoria, joined the exclusive list of MICHELIN-Starred eateries in Canada, as the 2023 MICHELIN Guide Toronto was announced Wednesday night.
Overall, the anonymous MICHELIN Guide inspectors awarded Stars to 15 restaurants, with Sushi Masaki Saito remaining atop that list with two MICHELIN Stars. The 2023 selection comprises 82 restaurants and 28 types of cuisine. Chefs and restaurant teams were honored during a ceremony at HISTORY in Toronto.
Frilu, which retained its one MICHELIN Star, and White Lily Diner, which earned a 2023 Bib Gourmand, were awarded the city’s first MICHELIN Green Stars in recognition for their leadership in sustainable gastronomy.
“Since the inaugural selection in Toronto last year, we have seen and felt the momentum grow in this culinary community,” said Gwendal Poullennec, the International Director of the MICHELIN Guides. “The passion, encouragement and determination has been evident, and we see the fruit of that labor today. We are very pleased to welcome Kappo Sato and Restaurant 20 Victoria into the family of MICHELIN-Starred restaurants. The famously anonymous Guide inspectors were also inspired by the commitment to sustainable gastronomy at Frilu and White Lily Diner.”
Here are the new one-MICHELIN-Star restaurants, with inspector notes from each (inspectors’ comments in full on the MICHELIN Guide website and mobile app):
One MICHELIN Star
Kappo Sato (Japanese cuisine)
Unlike the quiet ceremony of a sushi omakase or the formal structure of a kaiseki, this freewheeling tasting is driven solely by Chef Takeshi Sato, who swims in familiar culinary waters on his own terms. The room is a constant blur of motion thanks to a young team that hurries about preparing multiple courses at once. Sato is their seasoned guide, as he moves with intention, ever masterful with a knife, and works with an impressive bounty of ingredients, most of which are flown in from Japan. Soulful dashi broths weave in and out of view alongside clever courses like tempura fried mackerel with shiso or seared toro nigiri with Japanese green onions.
Restaurant 20 Victoria (Contemporary cuisine)
On a quiet stretch of downtown Toronto, Chef Julie Hyde is making her talents known in this tiny-but-mighty restaurant that captured the city’s heart the day it opened. Top-notch local produce, pristine seafood and refined sauce work make for a delicious trifecta on a seasonal tasting menu that boasts originality in spades. Recent highlights included smoked beets with oyster cream as well as grilled fish with beurre blanc and eel crumble. The dimly lit dining room is elegantly easy-going, as Hyde and her small team work with laser-like focus from a kitchen that’s simultaneously always in use and always pristine.
MICHELIN Green Star
Frilu (Contemporary cuisine)
Owner-established Willowolf Farm uses no-till methods to grow fruits and vegetables for Frilu to use throughout the seasons. Chef John-Vincent Troiano hand-picks produce every morning to ensure a truly sustainable farm-to-table experience. A composting program uses all kitchen waste to enrichen the farm’s soil. Canadian ingredients, bought from local markets and farmers, are used throughout the year.
White Lily Diner (Creative cuisine)
Their organic, no-till farm and greenhouses supply the diner (and more than 15 other restaurants) with produce. They raise chickens to supply the diner with eggs. Only hand tools are used on the farm. Insect netting and greenhouses mitigate insects and disease. An orchard was recently planted and is being cultivated.
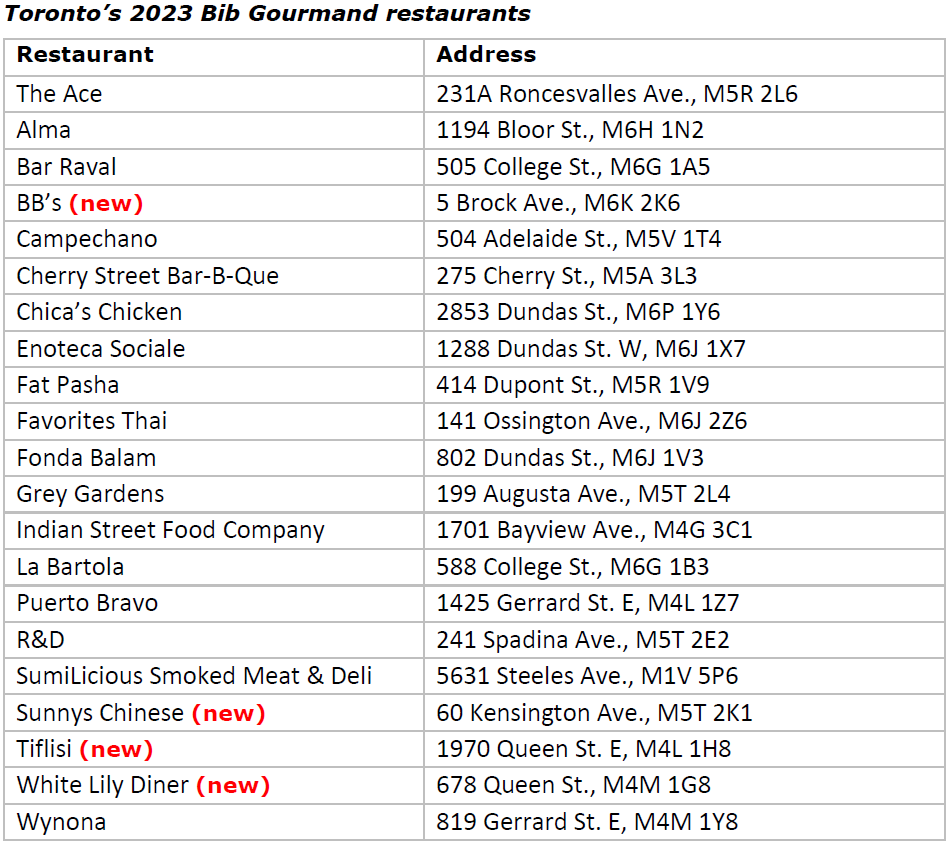
Bib Gourmand
The MICHELIN Guide inspectors added four restaurants to the Bib Gourmand list, which recognizes eateries for great food at a great value: BB’s, Sunnys Chinese, Tiflisi and White Lily Diner.
Special Awards
In addition to the Bib Gourmand and Stars, the Guide announced four special awards:
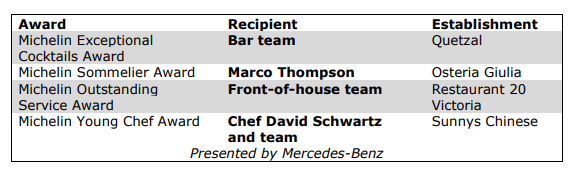
The MICHELIN Guide Ceremony is presented with the support of Capital One.
Hotels
The restaurants join the MICHELIN Guide selection of hotels, which features the most unique and exciting places to stay in Toronto and throughout the world. Each hotel has been chosen by MICHELIN Guide experts for its extraordinary style, service and personality — with options for all budgets — and each can be booked directly through the MICHELIN Guide website and app. The selection for Toronto features the city’s most spectacular hotels, including sustainability pioneers like 1 Hotel Toronto, standouts from the “Plus” collection like the Hazelton and the SoHo, cutting-edge boutiques like Ace Hotel and the Drake, and dependable luxury stalwarts like the Ritz-Carlton.
The MICHELIN Guide is a benchmark in gastronomy. Now it’s setting a new standard for hotels. Visit the MICHELIN Guide website, or download the free app for iOS and Android, to discover every restaurant in the selection and book an unforgettable hotel.

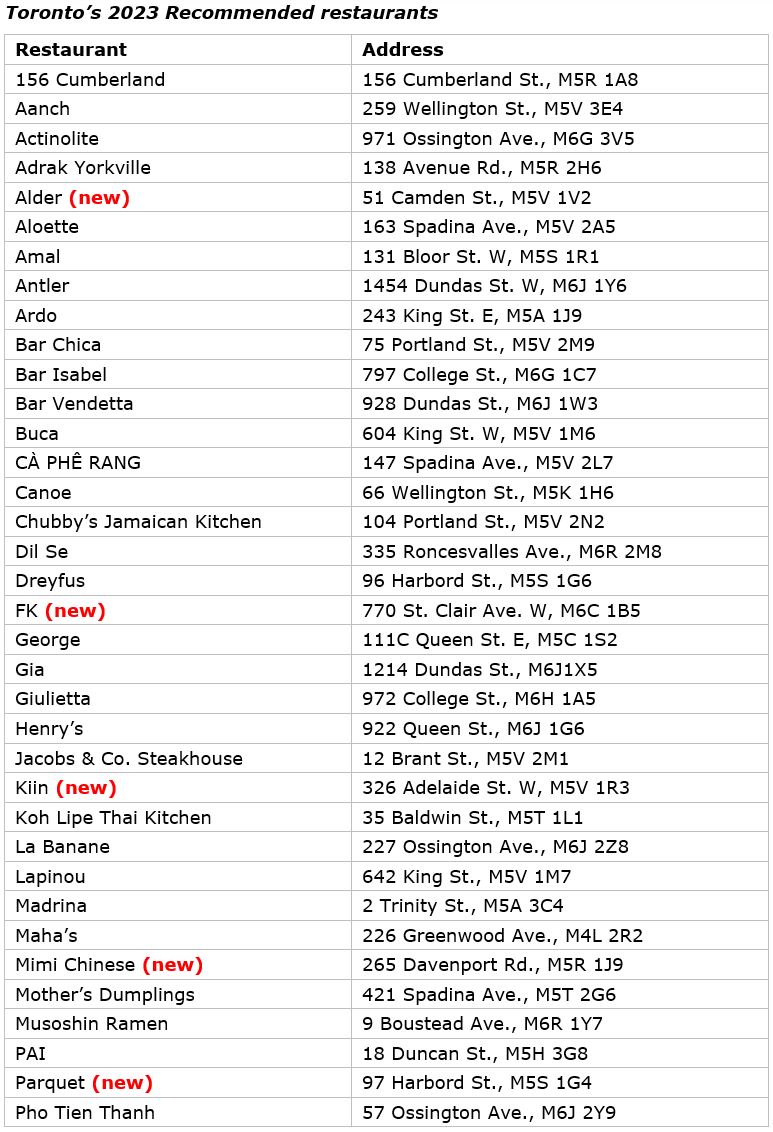
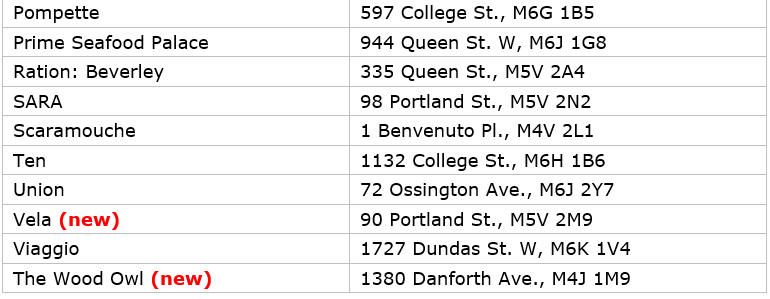
The 2023 MICHELIN Guide Toronto selection:
About Michelin North America, Inc.
Michelin, the leading mobility company, is working with tires, around tires and beyond tires to enable Motion for Life. Dedicated to enhancing its clients’ mobility and sustainability, Michelin designs and distributes the most suitable tires, services and solutions for its customers’ needs. Michelin provides digital services, maps and guides to help enrich travel and make them unique experiences. Bringing its expertise to new markets, the company is investing in high-technology materials, 3D printing and hydrogen, to serve a wide variety of industries — from aerospace to biotech. Headquartered in Greenville, South Carolina, Michelin North America has approximately 23,000 employees and operates 34 production facilities in the United States and Canada. (michelinman.com)
About Capital One
At Capital One we’re on a mission for our customers – bringing them best-in-class products, rewards, service, and experiences. Capital One is a diversified bank that offers products and services to individuals, small businesses and commercial clients. We use technology, innovation, and interaction to provide consumers with products and services to meet their needs. Through Capital One Dining and Capital One Entertainment, we provide our rewards cardholders with access to unforgettable experiences in the areas they’re passionate about, including dining, music and sports. Learn more at capitalone.com/dining and capitalone.com/entertainment.
For more information, contact:
Andrew Festa
Michelin North America
andrew.festa@michelin.com
Devon Gunn Capital One
devon.gunn@capitalone.com
Phone: 571-308-4762
