- Five restaurants receive a MICHELIN Star in inaugural selection
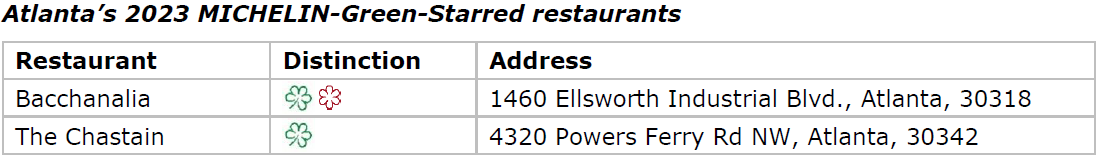
- City boasts two restaurants earning MICHELIN Green Star
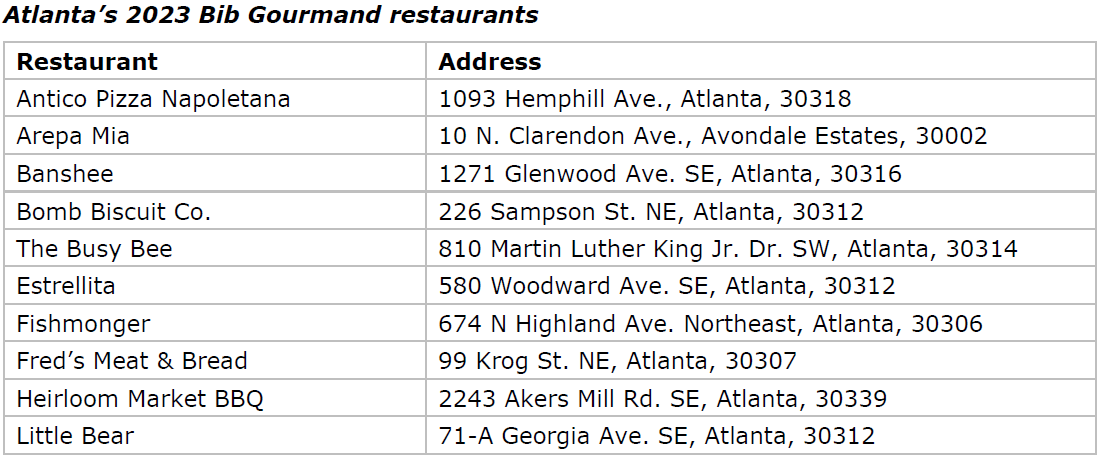
- 10 Bib Gourmand, plus four Special Awards, also revealed
- 45 total restaurants, 23 cuisine types reflected in Guide
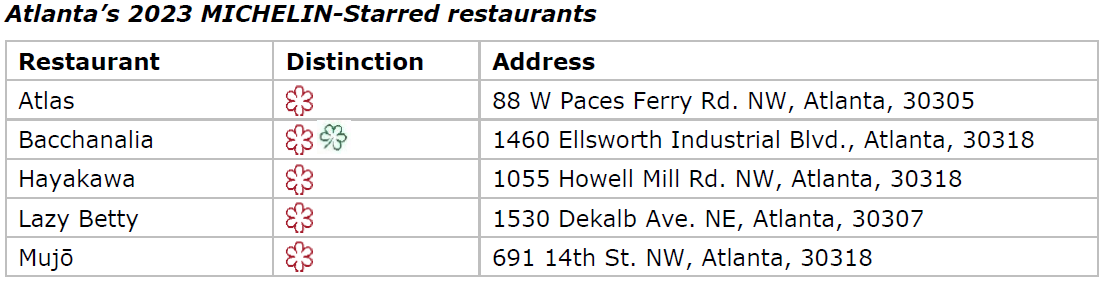
ATLANTA, Oct. 24, 2023 — The first MICHELIN Guide Atlanta has been revealed, and the selection features five one-MICHELIN-Star establishments and two MICHELIN Green Star eateries. Bacchanalia brought home one of each.
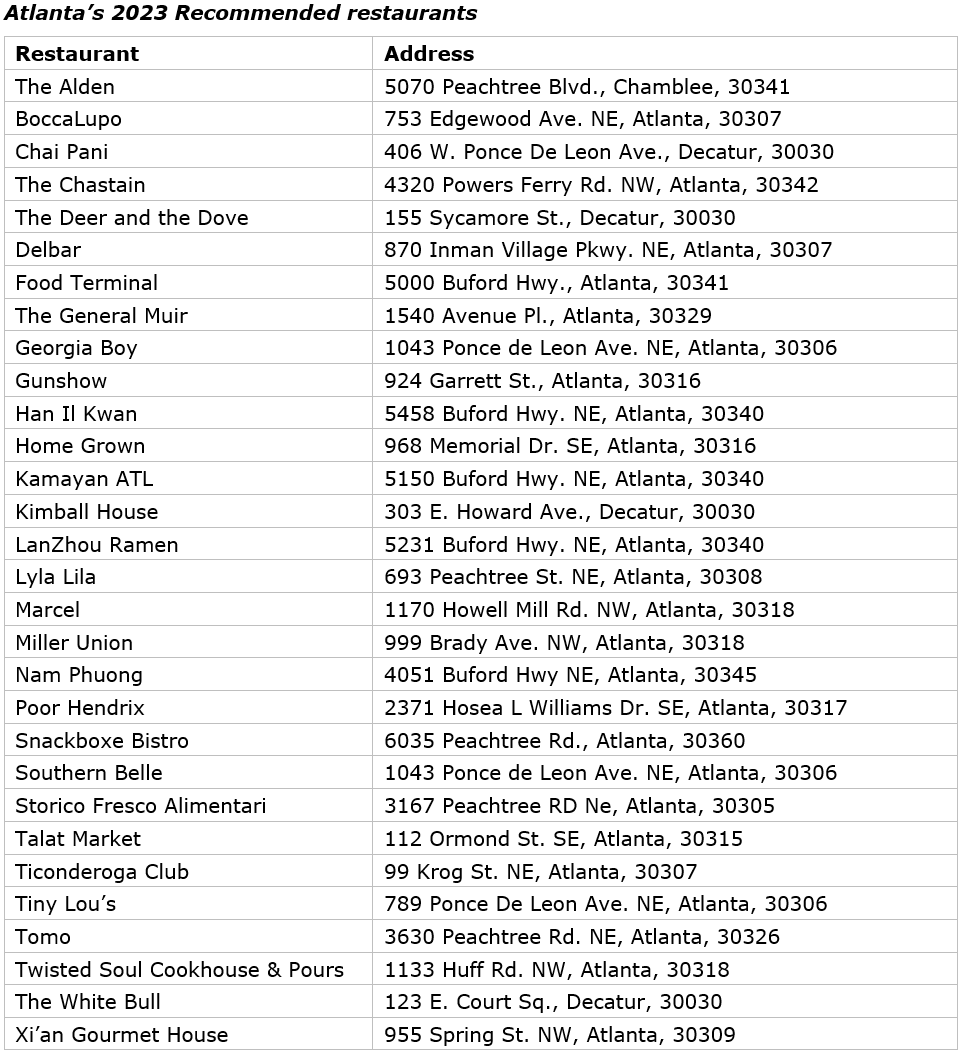
The full selection, including Bib Gourmand restaurants and Recommended eateries, totals 45 restaurants. Chefs and restaurant teams were honored Tuesday night at the Rialto Center for the Arts.
“Our famously anonymous inspectors enjoyed experiencing Atlanta and its dynamic culinary landscape,” said Gwendal Poullennec, the International Director of the MICHELIN Guides. “They came away impressed with the diverse offerings, as the selection of restaurants not only reflects a taste of the South, but also has a good deal of international flavor. There is so much for foodies to savor here. Whether they’re local residents or international travelers, they’re in for a treat!”
Here are the new one-MICHELIN-Star restaurants, with inspector notes from each (Inspectors’ comments in full on the MICHELIN Guide website and mobile app):
One MICHELIN Star
Atlas (American cuisine)
When the night calls for a grand celebration, few places fit quite like Atlas. Order à la carte from Chef Freddy Money’s seasonal American menu with European influences or celebrate with the tasting menu for dishes like tender lobster plated with smoked paprika butter sauce and heirloom summer squash, and poached halibut composed with a trio of beet preparations. Wagyu beef from Australia is a decadent end to the savory courses. Impressive cocktails, a cheese cart and whimsical desserts complete the well-rounded experience.
Bacchanalia (American cuisine)
Chefs/Owners Anne Quatrano and Clifford Harrison and Executive Chef Kai NaLampoon offer a multicourse prix fixe that involves a bit of flair, with some dishes arriving on carts or nestled inside glass cloches, and the cheese course is a wonderful surprise. Rather than an expected slice, the team presents a clever take with a crumbly oat date cake and a dot of black garlic sauce surrounded by rings of parmesan. Chilled lobster in a ponzu sauce with bright English peas and horseradish oil is also memorable, but it may just be the delicate grapefruit soufflé garnished with spicy pistachio crumble and rose crème anglaise that takes the cake.
Hayakawa (Japanese cuisine)
A local legend for his Japanese cuisine on Buford Highway, Chef Atsushi Hayakawa has begun a new chapter in West Midtown. The meal is a procession of small courses and hews toward the structure of kaiseki. After items such as an appetizer trio with clear fish soup, scallop sashimi with miso-mustard sauce and simmered monkfish, it’s time for sushi. The chef crafts nigiri from imported fish that needs little embellishment and is amply sized in the tradition of Hokkaido style in deference to the chef’s hometown.
Lazy Betty (Contemporary cuisine)
Chef Ron Hsu and Chef Aaron Phillips oversee a contemporary tasting menu with clever flavor combinations that highlight regional ingredients. Causa is given a Southern slant with sweet Georgia shrimp, avocado purée and potato foam infused with aji amarillo pepper, while seared Hudson Valley foie gras is sided by Granny Smith apple, sweet potato and dots of pumpkin butter. From the pre-dessert lemon sherbet with a coconut crumble to the elegant rosewater panna cotta, they impress to the end.
Mujō (Japanese cuisine)
Mujō is an intimate setting with a moody elegance. This is the domain of Chef J. Trent Harris and his skilled team who make all feel well cared for. Here, tradition has been replaced with a rollicking good time, where the always-surprising interpretation of omakase begin with an array of zensai, like a morsel of Florida cobia grilled over binchotan, dressed with a red miso sauce and some local pattypan squash. After some cooked bites, it’s time for the raw. Nigiri needs little to impress, while supplemental dishes offer the likes of Hokkaido hair crab, tosazu and mozuku.
MICHELIN Green Star
Bacchanalia (American cuisine)
Chefs own and operate Summerland Farm in Cartersville, Georgia, where they grow much of their produce and harvest eggs. They also have a composting program, and they feed chickens with vegetable scraps.
The Chastain (American cuisine)
Chef Christopher Grossman’s menu changes often, depending on what’s available from local farm partners and in his onsite regenerative-farmed garden. The Chastain recently joined Georgia Organics to help quantify local and organic food purchases. The team composts on site and also uses a compost company to reduce landfill waste. They also recycle glass and use compostable carryout containers.
Bib Gourmand
The MICHELIN Guide inspectors gave 10 restaurants the Bib Gourmand distinction, which recognizes eateries for great food at a great value: Antico Pizza Napoletana, Arepa Mia, Banshee, Bomb Biscuit Co., The Busy Bee, Estrellita, Fishmonger, Fred’s Meat & Bread, Heirloom Market BBQ, and Little Bear.
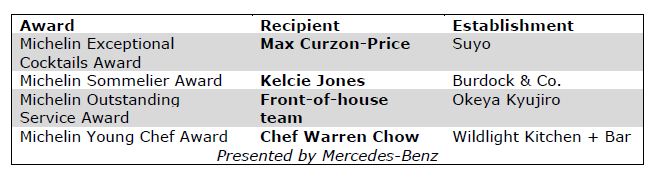
MICHELIN Special Awards
In addition to the Bib Gourmand and Stars, the Guide announced four Special Awards:

The MICHELIN Guide Ceremony is presented with the support of Capital One.
Hotels
The restaurants join the MICHELIN Guide selection of hotels, which features the most unique and exciting places to stay in Georgia and throughout the world.
Each hotel in the selection has been chosen by MICHELIN Guide experts for its extraordinary style, service and personality — with options for all budgets — and each can be booked directly through the MICHELIN Guide website and app. The selection for Georgia features the state’s most spectacular hotels, including design-forward boutiques like the Clermont in Atlanta and the Perry Lane in Savannah, standouts from our “Plus” collection like the intimate Stonehurst Place, music-inspired college-town haunts like Graduate Athens, and dependable luxury-boutique stalwarts like the Thompson and the Andaz.
The MICHELIN Guide is a benchmark in gastronomy. Now it’s setting a new standard for hotels. Visit the MICHELIN Guide website, or download the free app for iOS and Android, to discover every restaurant in the selection and book an unforgettable hotel.


About Michelin North America, Inc.
Michelin, the leading mobility company, is working with tires, around tires and beyond tires to enable Motion for Life. Dedicated to enhancing its clients’ mobility and sustainability, Michelin designs and distributes the most suitable tires, services and solutions for its customers’ needs. Michelin provides digital services, maps and guides to help enrich travel and make them unique experiences. Bringing its expertise to new markets, the company is investing in high-technology materials, 3D printing and hydrogen, to serve a wide variety of industries — from aerospace to biotech. Headquartered in Greenville, South Carolina, Michelin North America has approximately 23,000 employees and operates 34 production facilities in the United States and Canada. (michelinman.com)
About Capital One
At Capital One we’re on a mission for our customers – bringing them best-in-class products, rewards, service, and experiences. Capital One is a diversified bank that offers products and services to individuals, small businesses and commercial clients. We use technology, innovation, and interaction to provide consumers with products and services to meet their needs. Through Capital One Dining and Capital One Entertainment, we provide our rewards cardholders with access to unforgettable experiences in the areas they’re passionate about, including dining, music and sports. Learn more at capitalone.com/dining and capitalone.com/entertainment.
For more information, contact:
Andrew Festa
Michelin North America
andrew.festa@michelin.com
Devon Gunn
Capital One
devon.gunn@capitalone.com
Phone: 571-308-4762











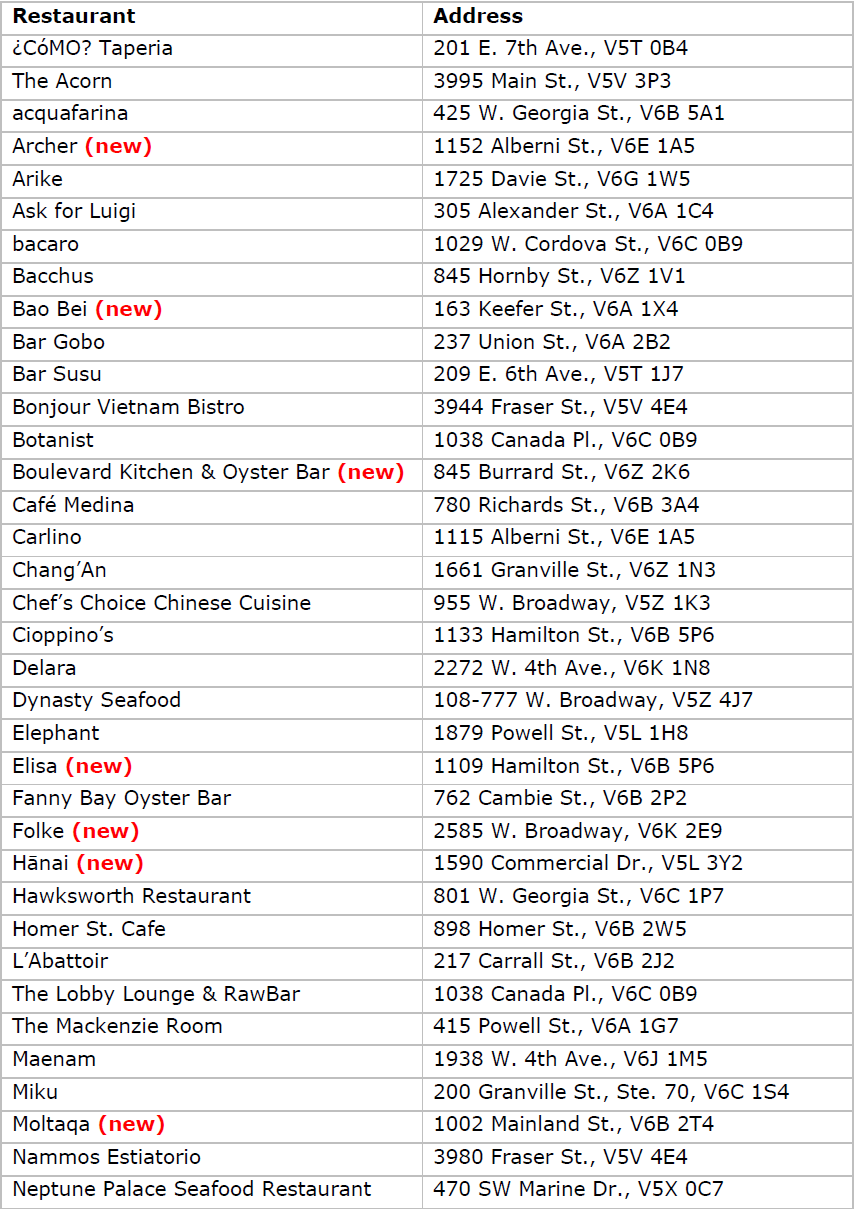
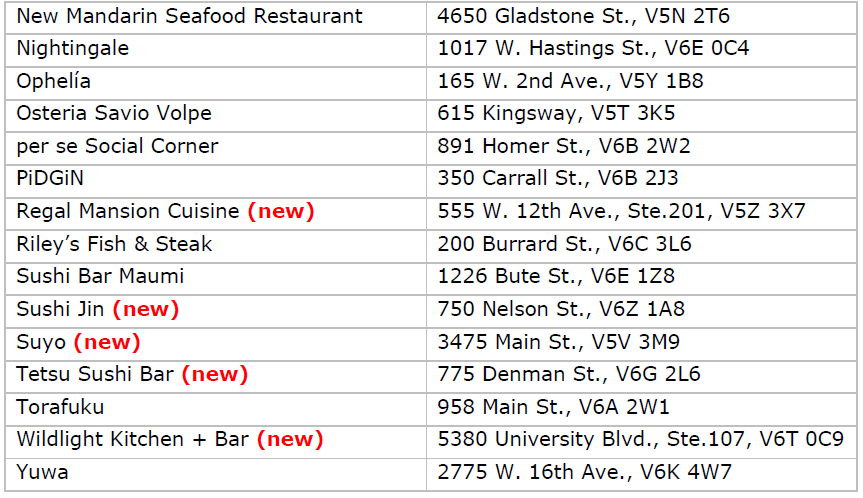
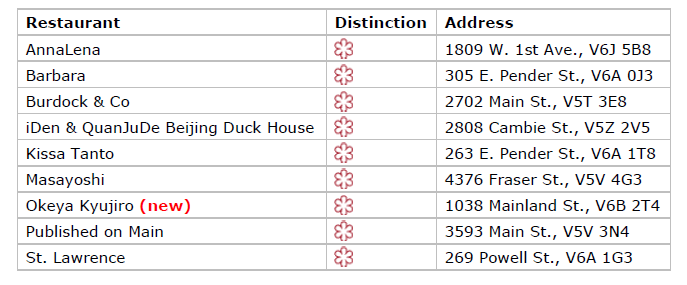 Vancouver’s 2023 Bib Gourmand restaurants
Vancouver’s 2023 Bib Gourmand restaurants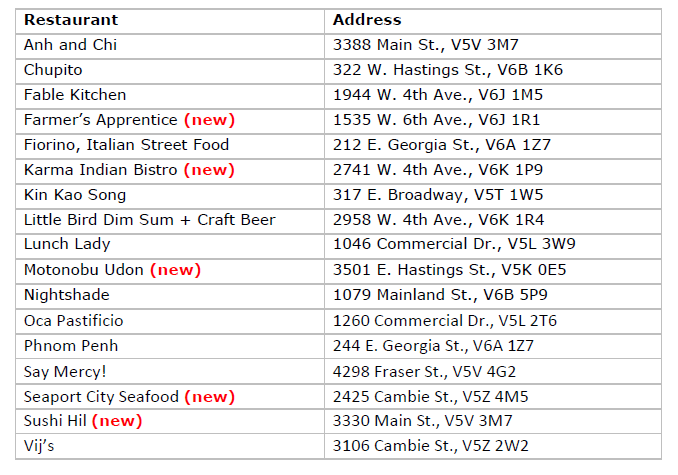 Vancouver’s 2023 Recommended restaurants
Vancouver’s 2023 Recommended restaurants