Top 100 Companies Identified by LexisNexis Intellectual Property Solutions Based on Quantifiable Improvement in Patent Portfolios
New York, NY – March 4, 2026 – LexisNexis® Legal & Professional today announced the “Top 100 Global Innovators” for 2026, the companies around the world that are driving innovation in the global economy, based on quantifiable improvement in the patent portfolios held by those companies over the last two years. Representing 18 countries, the top 100 companies were identified through an analysis by LexisNexis® Intellectual Property Solutions using its proven Patent Asset Index methodology across its database of more than 17 million global patent families.
“Innovation can sometimes feel like an abstract concept that is hard to measure and even harder to improve. Happily, patent analytics can provide a relevant and impactful gauge for evaluating innovation from companies across all industries, technologies, and sizes,” said Marco Richter, Managing Director and Chief Commercial Officer for LexisNexis Intellectual Property Solutions. “LexisNexis® Patent Asset Index provides an objective apples-to-apples framework for analyzing innovation leadership based on changes in the strength of each company’s patent assets. This goes beyond simply counting patents. The Top 100 Global Innovators have showcased true leadership by advancing the world’s knowledge and developing technologies and services that help shape a better future.”
Key Insights:
Semiconductors Take the Lead: Having dominated the 2025 ranking by company count, pharmaceuticals cede the top position this year to the semiconductor industry, which leads with 14 honorees. Pharmaceuticals now rank second with 13 companies, followed by Chemicals and Materials with 12 entrants.
Innovators Span the Globe: For the fifth consecutive year, US-based companies dominated the Top 100 Global Innovators with 46 honorees. US-based companies on the list excel in Pharmaceuticals and Medical Technology, while Asia dominates representation in Chemicals and Materials. Notably, within the Asian region there is balanced representation from China, Korea, and Japan, each represented by seven companies. EMEA scores highest in the Automotive sector with strong representation from Germany.
Top 100 Global Innovators by Industry Sector
|
Industries |
Americas |
Asia |
EMEA |
Grand Total |
|
Semiconductors |
6 |
5 |
3 |
14 |
|
Pharmaceuticals |
10 |
– |
3 |
13 |
|
Chemicals and Materials |
1 |
6 |
5 |
12 |
|
Consumer Goods |
4 |
4 |
3 |
11 |
|
Information Technologies |
6 |
2 |
3 |
11 |
|
Engineering |
4 |
1 |
5 |
10 |
|
Medical Technologies |
7 |
– |
– |
7 |
|
Technology R&D |
5 |
1 |
– |
6 |
|
Automotive |
– |
1 |
5 |
6 |
|
Electronics |
2 |
4 |
– |
6 |
|
Biotechnologies |
2 |
– |
– |
2 |
|
Appliances |
– |
1 |
– |
1 |
|
Conglomerates |
– |
1 |
– |
1 |
|
Grand Total |
47 |
26 |
27 |
100 |
New Entrants Range from Startups to Industry Giants: The report introduces 21 new entrants to the list, reflecting the increasingly global and cross-sector nature of breakthrough innovation. From small companies to global conglomerates, those new companies include innovators such as Adeia and Flagship Pioneering (US), Airbus and Safran (EMEA), and Fujitsu and LONGi Green Energy (Asia), illustrating the breadth of momentum, as they span advanced engineering, next-generation electronics, clean energy technologies, and life sciences.
Intellectual Property Drives Corporate Strategy: The report emphasizes the importance of high-quality, impactful patent portfolios in driving competitive advantage and revenue. For example, IBM reflects a value-driven approach, while Adeia focuses on acquiring strategic portfolios for targeted growth, and Huawei and InterDigital demonstrate how strong portfolios underpin sustainable licensing revenue.
Smaller Companies Demonstrate Value of Patent Assets: Smaller, specialist players such as Acuitas Therapeutics, Strong Force IP, Ofinno, and Magic Leap illustrate how focused capabilities and high-value IP can create outsized strategic relevance. This dynamic is reinforced by the combination of two companies on this year’s Top 100 list, as Eli Lilly acquired venture-backed Orna Therapeutics in February.
US and China Dominate Academic Arena: The academic innovation leaders are mostly made up of US and Chinese institutions, highlighting their central role in high-impact research. The United States features leading research institutions such as the Broad Institute, MIT, Harvard, and the University of California, while China shows strong depth across multiple universities and research labs. Europe is solely represented by Germany’s Fraunhofer, underscoring continued strength in applied research and technology transfer.
Five-Year Innovation Momentum Shows Both Change and Continuity: Over five years, Top 100 recognitions have been awarded to 190 companies across 21 countries, led by the United States, followed by China and Germany.
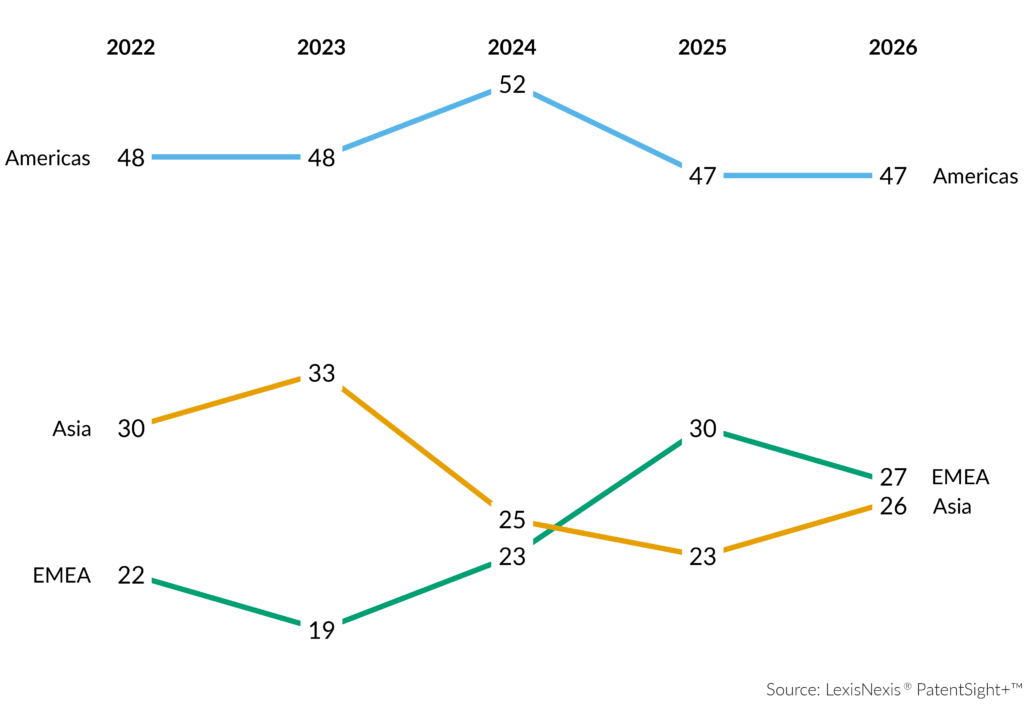
Pharmaceuticals stood out with 87 recognitions, ahead of Semiconductors and Information Technologies. Biotechnologies showed the highest recurrence and stability, while Engineering reflected greater volatility with the lowest average repeat rate of companies.
New: Inventor Network Analysis: This year’s report introduces a new framework for analyzing inventor networks, showing how collaboration patterns drive innovation performance. By mapping co-invention relationships, it identifies teams, key connectors, and knowledge flows. For IP professionals, this framework provides actionable insights to support competitive intelligence, identification of critical inventors, organizational benchmarking, and enhanced M&A due diligence.
The Top 100 Global Innovators list was released as part of the Innovation Momentum 2026: The Global Top 100 report.
Top 100 Global Innovators in Alphabetical Order with Global Headquarters and Industry Sector
|
Patent Owner |
HQ |
Industry |
|
10x Genomics |
US |
Biotechnologies |
|
Acuitas Therapeutics |
CA |
Pharmaceuticals |
|
Adeia* |
US |
Technology R&D |
|
AGCO* |
US |
Engineering |
|
Airbus Group* |
NL |
Engineering |
|
Align Technology |
US |
Medical Technologies |
|
Alnylam Pharmaceuticals |
US |
Pharmaceuticals |
|
Alphabet |
US |
Information Technologies |
|
Amazon |
US |
Information Technologies |
|
Amgen |
US |
Pharmaceuticals |
|
Apple |
US |
Electronics |
|
Applied Materials |
US |
Semiconductors |
|
ARAMCO |
SA |
Chemicals and Materials |
|
ASM |
NL |
Semiconductors |
|
ASML |
NL |
Semiconductors |
|
AutoStore |
NO |
Information Technologies |
|
BASF |
DE |
Chemicals and Materials |
|
Becton, Dickinson |
US |
Medical Technologies |
|
BioNTech |
DE |
Pharmaceuticals |
|
Boeing |
US |
Engineering |
|
Bosch |
DE |
Automotive |
|
Bristol-Myers Squibb |
US |
Pharmaceuticals |
|
British American Tobacco |
GB |
Consumer Goods |
|
CATL |
CN |
Chemicals and Materials |
|
Chengdu Qinchuan IoT |
CN |
Technology R&D |
|
Coupang |
US |
Information Technologies |
|
Daikin |
JP |
Appliances |
|
Deere & Co |
US |
Engineering |
|
Dyson* |
SG |
Consumer Goods |
|
E Ink* |
TW |
Electronics |
|
Edwards Lifesciences |
US |
Medical Technologies |
|
Eli Lilly |
US |
Pharmaceuticals |
|
Ericsson |
SE |
Information Technologies |
|
Flagship Pioneering* |
US |
Technology R&D |
|
Fujitsu* |
JP |
Conglomerates |
|
Gilead Sciences |
US |
Pharmaceuticals |
|
Honor Device* |
CN |
Electronics |
|
Huawei |
CN |
Information Technologies |
|
Hyundai Motor |
KR |
Automotive |
|
IBM |
US |
Information Technologies |
|
Illumina |
US |
Biotechnologies |
|
Intel |
US |
Semiconductors |
|
InterDigital |
US |
Technology R&D |
|
Intl. Flavors & Fragrances |
US |
Chemicals and Materials |
|
Intuitive Surgical |
US |
Medical Technologies |
|
Japan Tobacco |
JP |
Consumer Goods |
|
JFE Holdings |
JP |
Engineering |
|
JinkoSolar* |
CN |
Semiconductors |
|
Johnson & Johnson |
US |
Pharmaceuticals |
|
KLA |
US |
Semiconductors |
|
Krones* |
DE |
Engineering |
|
KT&G |
KR |
Consumer Goods |
|
Lam Research |
US |
Semiconductors |
|
LG Chem |
KR |
Chemicals and Materials |
|
LG Electronics |
KR |
Electronics |
|
LONGi Green Energy* |
CN |
Semiconductors |
|
Magic Leap |
US |
Electronics |
|
Masimo |
US |
Medical Technologies |
|
MediaTek |
TW |
Semiconductors |
|
Meta |
US |
Information Technologies |
|
Moderna Therapeutics |
US |
Pharmaceuticals |
|
Nestlé |
CH |
Consumer Goods |
|
Nike |
US |
Consumer Goods |
|
Nippon Steel* |
JP |
Chemicals and Materials |
|
Nvidia |
US |
Semiconductors |
|
Ocado |
GB |
Information Technologies |
|
Ofinno |
US |
Technology R&D |
|
OMV Group |
AT |
Chemicals and Materials |
|
Orna Therapeutics* |
US |
Pharmaceuticals |
|
P&G |
US |
Consumer Goods |
|
Philip Morris |
US |
Consumer Goods |
|
Qualcomm |
US |
Semiconductors |
|
Regeneron |
US |
Pharmaceuticals |
|
Roche |
CH |
Pharmaceuticals |
|
Rolls-Royce |
GB |
Engineering |
|
ROMTech* |
US |
Medical Technologies |
|
RTX |
US |
Engineering |
|
Safran* |
FR |
Engineering |
|
Saint-Gobain |
FR |
Chemicals and Materials |
|
Samsung |
KR |
Electronics |
|
Samsung SDI |
KR |
Chemicals and Materials |
|
Sanofi |
FR |
Pharmaceuticals |
|
Schaeffler* |
DE |
Automotive |
|
SharkNinja* |
US |
Consumer Goods |
|
Shin-Etsu* |
JP |
Chemicals and Materials |
|
SK Innovation |
KR |
Chemicals and Materials |
|
Snap |
US |
Information Technologies |
|
Strong Force Innovation* |
US |
Technology R&D |
|
Stryker |
US |
Medical Technologies |
|
Techtronic |
HK |
Consumer Goods |
|
Tetra Laval* |
CH |
Consumer Goods |
|
thyssenkrupp |
DE |
Engineering |
|
Tokyo Electron |
JP |
Semiconductors |
|
Topsoe |
DK |
Chemicals and Materials |
|
TSMC |
TW |
Semiconductors |
|
Valeo* |
FR |
Automotive |
|
VW Group |
DE |
Automotive |
|
ZEISS |
DE |
Semiconductors |
|
ZF |
DE |
Automotive |
|
ZTE |
CN |
Information Technologies |
* Denotes first-time inclusion in the Global Top 100 Innovators list.
The full report is available for download at http://www.lexisnexisip.com/most-innovative-companies-2026
About the Methodology
LexisNexis® conducted an analysis across its database of more than 17 million global patent families to identify companies that are leaders in global innovation. The methodology was based on the LexisNexis Patent Asset Index, which was used to measure the strength and quality of a company’s patent assets. The analysis tracked exceptional shifts in patent portfolio quality over the last two years to identify companies as the “Top 100 Global Innovators” in this year’s “Innovation Momentum 2026” report.
About LexisNexis® Legal & Professional
LexisNexis® Legal & Professional provides AI-powered legal, regulatory, business information, analytics, and workflows that help customers increase their productivity, improve decision-making, achieve better outcomes, and advance the rule of law around the world. As a digital pioneer, the company was the first to bring legal and business information online with its Lexis® and Nexis® services. LexisNexis Legal & Professional, which serves customers in more than 150 countries with 11,900 employees worldwide, is part of RELX, a global provider of information-based analytics and decision tools for professional and business customers.
About LexisNexis® Intellectual Property Solutions
LexisNexis® Intellectual Property Solutions brings clarity to innovation for businesses worldwide. We enable innovators to accomplish more by helping them make informed decisions, be more productive, comply with regulations, and ultimately achieve a competitive advantage for their business. Our broad suite of workflow and analytics solutions (LexisNexis® PatentSight+ , LexisNexis® Classification, LexisNexis® TechDiscovery, LexisNexis® IPlytics
, LexisNexis® Classification, LexisNexis® TechDiscovery, LexisNexis® IPlytics , LexisNexis PatentOptimizer®, LexisNexis PatentAdvisor®, and LexisNexis TotalPatent One®, LexisNexis® IP DataDirect), enables companies to be more efficient and effective at bringing meaningful innovations to our world. We are proud to directly support and serve these innovators in their endeavors to better humankind.
, LexisNexis PatentOptimizer®, LexisNexis PatentAdvisor®, and LexisNexis TotalPatent One®, LexisNexis® IP DataDirect), enables companies to be more efficient and effective at bringing meaningful innovations to our world. We are proud to directly support and serve these innovators in their endeavors to better humankind.
Media Contact:
Andrew Weinstein
Andrew.Weinstein@LexisNexis.com
LexisNexis | Intellectual Property Solutions
Bringing clarity to innovation
Contact Information
Name: Andrew Weinstein
Email: andrew.weinstein@lexisnexis.com
Job Title: PR Consultant
Advertising Watchdogs Find Dorel’s AI-Related Claims Supported; Recommends Improvements to COPPA Notice and Consent Practices

New York, NY – March 3, 2026 – In a joint inquiry, BBB National Programs’ National Advertising Division and Children’s Advertising Review Unit (CARU) found that Dorel Juvenile Group, Inc.’s express claims regarding the performance, functionality, and privacy protections of its CryAssist technology used in the Maxi‑Cosi Sibia Bassinet and Starling Smart Bassinet are supported.
technology used in the Maxi‑Cosi Sibia Bassinet and Starling Smart Bassinet are supported.
However, CARU recommended Dorel implement changes to its online privacy practices to ensure compliance with the Children’s Online Privacy Protection Act (COPPA), specifically with respect to direct notice to parents and verifiable parental consent.
Dorel markets a collection of connected baby nursery products under the MaxiCosi brand, including the Sibia Bassinet with CryAssist Audio Monitor and the Starling Smart Bassinet. These products include CryAssist technology developed by Zoundream AG, a Swiss-based company.
technology developed by Zoundream AG, a Swiss-based company.
As part of their ongoing monitoring programs, the National Advertising Division (NAD) and CARU brought this inquiry to review certain AI-related advertising claims as well as data privacy practices regarding children, as the products collect audio data from children under 13 and include AI-based features marketed to parents.
At issue for NAD were Dorel’s claims that its CryAssist technology uses AI to translate infant cries into categories (sleepy, fussy, gassy, agitated, or hungry) and that cry data is anonymized, encrypted, and processed securely.
In support of its claims, Dorel provided peer-reviewed published research documenting and validating the underlying AI model, as well as evidence demonstrating calibration and performance of the technology in the devices.
Based on that evidence, NAD concluded that Dorel supported the claim “Our groundbreaking CryAssist technology uses AI to translate your little one’s cries, letting you know if they might be sleepy, fussy, gassy, agitated, or hungry.”
technology uses AI to translate your little one’s cries, letting you know if they might be sleepy, fussy, gassy, agitated, or hungry.”
Regarding the claim “Each of CryAssist’s response-based features are optional, ensuring control is always in your hands,” NAD found that the claim that this feature is “optional” and that users are in “control” was also supported.
In support of the claim, “With cries and cry data kept anonymous and encrypted on our cloud, private moments are kept between you and baby,” Dorel provided an explanation and documentation of its data collection and retention practices related to cry sounds. Based on the evidence, NAD found the claim supported.
At issue for CARU was whether Dorel’s data collection practices comply with COPPA and CARU’s Privacy Guidelines. CARU found that Dorel maintains reasonable security measures, limits data collection to information necessary to support the service, and does not use children’s data for undisclosed secondary purposes.
However, CARU concluded that Dorel’s current practices demonstrate deficiencies with respect to COPPA’s notice-based requirements. Specifically, those deficiencies are the absence of a compliant online privacy policy and direct notice to parents, and the lack of a verifiable parental consent mechanism. CARU therefore recommended that Dorel implement these required notice and consent procedures.
In its advertiser statement, Dorel stated that it “will comply with the decision of the NAD and CARU.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. This press release shall not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than 20 globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, promote fair competition for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. The National Advertising Division reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and promoting fair competition for business.
Contact Information
Name: Jennie Rosenberg
Email: press@bbbnp.org
Job Title: Media Relations
National Advertising Division Recommends OLLY Modify “Calm & Relaxed” Claims for Kids Chillax Supplement

New York, NY – March 2, 2026 – In a challenge brought by competitor Bayer HealthCare LLC, BBB National Programs’ National Advertising Division recommended that OLLY PBC modify claims that its Kids Chillax dietary supplement supports a calm and relaxed mood “for ages 7+” to avoid conveying the message that Chillax reduces children’s activity levels.
After a decision by the National Advertising Division (NAD) (#7350) and appeal to the National Advertising Review Board (#7350-337), this matter was reopened after NAD agreed to consider new evidence submitted by OLLY.
In the original case, NAD concluded that Olly’s study was not a good fit for the challenged express claims that Kids Chillax can calm kids and make them stay engaged because, among other reasons, NAD found that the assessments used to assess anxiety levels in the study were not reliable for the youngest participants in the study.
In this reopened proceeding, OLLY submitted new evidence after modifying its advertising, including removing imagery of pandas, changing the age recommendation from 4+ to 7+, removing the language “peaceful pals” and “gently calm little minds while helping kiddos stay engaged,” and removing the claim “L-Theanine. Captain calm. This amino acid supports a relaxed state of mind.”
In support of its modified claims targeting children ages seven and older, OLLY submitted a post-hoc analysis of the Chillax Study limited to the subgroup of children ages seven and fifteen.
The Chillax Study was comprised of three scales that that measured the emotional state of the subjects. NAD found that the post-hoc subgroup analysis was unreliable as to two of the scales because their results were not consistent with the results of the larger study from the initial NAD case. Measures that were statistically significant in the full study were not statistically significant in the subgroup, and measures that were not statistically significant in the full study became statistically significant in the subgroup.
NAD further found that one of the scales used in the Chillax Study consistently showed statistical significance. However, this scale assessed anxiety rather than activity levels and therefore could not support the implied message that Kids Chillax reduces children’s activity levels.
Accordingly, NAD recommended that OLLY modify its Kids Chillax advertising to avoid conveying the unsupported message that Chillax reduces activity levels. In particular, NAD recommended that the following claims be modified to clearly and conspicuously disclose that “calm and relaxed” does not refer to a reduction in activity levels:
- OLLY’s Kids Chillax “supports a calm and relaxed mood*” “*for ages 7+”
- “Z is for Zen: These delightful gummies are formulated to support a calm & relaxed mood. That’s our kind of peace of mind.”
- Kids Chillax is “formulated to support a calm & relaxed mood.”
In its advertiser statement, OLLY stated it “will comply with the recommendation that references to “calm and relaxed” avoid implying a reduction in activity levels.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. Per NAD/NARB Procedures, this release may not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than 20 globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, promote fair competition for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. The National Advertising Division reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and promoting fair competition for business.
Contact Information
Name: Jennie Rosenberg
Email: press@bbbnp.org
Job Title: Media Relations
National Advertising Division Will Refer Laifen to Federal and State Regulatory Authorities for Failure to Comply with Recommendations

New York, NY – February 26, 2026 – Following a BBB National Programs’ National Advertising Division compliance challenge brought by Dyson, Inc., Shuye Technology Ltd. d/b/a Laifen will be referred to the relevant federal agency and state attorneys general for failure to comply with National Advertising Division (NAD) recommendations.
In an NAD case from January 2024, Laifen agreed to permanently discontinue claims on its website and on social media that its Swift, Swift SE, Swift Special, and Swift Premium hair dryers are the “fastest.” In reliance on Laifen’s representation that the challenged claims had been permanently discontinued, the NAD did not review the claims on their merits (Case #7275).
In October 2024, Dyson requested that NAD open a compliance inquiry based on its concerns about substantially similar claims for the Swift hair dryers. NAD inquired about Laifen’s efforts to comply with NAD’s recommendations. After close consultation with NAD, Laifen discontinued all of the challenged claims. Consequently, NAD concluded that no further action was required and NAD closed the compliance proceeding.
Dyson later contacted NAD concerning additional, numerous noncompliant advertisements appearing on Laifen’s website and on social media. Despite frequent outreach attempts to Laifen and separate unsuccessful attempts to have the advertisements removed from the various platforms with which NAD has a reporting relationship, the noncompliant advertisements remain live and unmodified.
As a result, NAD determined that Laifen has not undertaken a good faith effort to comply with NAD’s recommendations and will refer Laifen to the appropriate regulatory authorities, including the relevant state attorneys general, pursuant to Section 8.1(B)(3)(c)(ii) of the NAD/NARB Procedures.
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. Per NAD/NARB Procedures, this release may not be used for promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than 20 globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create fair competition for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and create fair competition for business.
Contact Information
Name: Jennifer Rosenberg
Email: press@bbbnp.org
Job Title: Media Relations
National Advertising Division Recommends Monarch Money Discontinue Certain Money Management Influencer Claims

New York, NY – February 26, 2026 – Following a review of social media influencer advertising for the Monarch Money app, BBB National Programs’ National Advertising Division recommended that Monarch Money, Inc. discontinue certain express consumer survey-based outcome claims.
Monarch is a personal finance technology company that provides a platform to help individuals and families manage their finances. Its Monarch Money service is offered on a paid subscription basis.
At issue for the National Advertising Division (NAD) were claims based upon the results of a consumer survey of Monarch Money users. All of the claims relied, at least in part, on yes-or-no responses to the survey, which did not include an “I don’t know” or “not sure” response option. NAD found that, in this context, survey questions formatted in this matter can inflate affirmative responses and undermine the reliability of the resulting data.
Members Save $200 Per Month Claim
Monarch based its claim, “On average, members save $200 per month using Monarch,” on a survey question that first asked respondents, with only “Yes” or “No” options, whether joining Monarch helped them save more or reduce unnecessary spending. Those who answered “Yes” were then asked to estimate how much they had saved or cut in unnecessary spending per month, which produced a monthly figure exceeding $215.
NAD found the follow-up question ambiguous because it combined two distinct behaviors—saving more and reducing spending—without clarifying which figure respondents should report. NAD also noted that Monarch’s app displays “Savings” as net cash flow (income minus expenses), which may have led some respondents to report that figure rather than actual increased savings. Based on these and other concerns, NAD concluded the claim is not supported and recommended it be discontinued.
Clearer Picture of their Money
As support for the claim “80% of members say Monarch gives them a clearer picture of their money,” Monarch relied on a survey question asking whether, “as a result of joining Monarch,” they had “[l]earned where your money is going better than you understood before.”
NAD found the claim communicates a broad message about gaining a clearer understanding of one’s overall financial situation, including more than just spending. The survey, however, measured only whether users believed they had improved visibility into where their money was going. Given the breadth of the claim relative to the narrow survey question and the forced-choice yes-or-no format, NAD determined the claim is not supported and recommended it be discontinued.
Improved Money Conversations
In support of the claim “7 in 10 couples say Monarch improved their money conversations with their partner,” Monarch relied on a survey question asking whether, “as a result of joining Monarch,” users “had better financial conversations with a partner.” NAD found the claim communicates a shared perception between two members of a couple, yet the evidence consists solely of individual self-reports and did not establish whether respondents were in couples or whether they interpreted “partner” consistently with the advertising claim.
Given these limitations and the forced-choice yes-or-no format, NAD determined the claim is not supported and recommended it be discontinued.
Feel More in Control of Finances
Monarch based the claim “8 in 10 feel more in control of their finances with Monarch” on a survey question asking “Has joining Monarch changed the way you feel about finances at all … I feel more in control.” Given the forced yes-or-no response methodology, NAD concluded the claim is not supported and recommended it be discontinued.
In its advertiser statement, Monarch stated that it “supports NAD’s self-regulatory process and will comply with NAD’s decision.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. Per NAD/NARB Procedures, this release may not be used for promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than 20 globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create fair competition for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and promoting fair competition for business.
Contact Information
Name: Jennifer Rosenberg
Email: press@bbbnp.org
Job Title: Media Relations
In National Advertising Division Challenge, Midi Health Permanently Discontinues Challenged Claims for Menopause Care Services

New York, NY, February, 25, 2026 – As part of its monitoring program, BBB National Programs’ National Advertising Division challenged express and implied advertising claims made by Midi Health, Inc. for its perimenopause and menopause services.
Midi Health is a virtual healthcare provider offering services to women experiencing symptoms associated with perimenopause, menopause, and related midlife health concerns. At issue for the National Advertising Division (NAD) was an Instagram post stating “Ready to say goodbye to hot flashes, weight gain, insomnia and mood swings? Join the 91% of patients who find relief within 2 months” and the related implied claim that patients experience significant symptom relief within two months and nearly all patients will see an elimination of key menopausal symptoms.
During the inquiry, Midi Health informed NAD that it had permanently discontinued the challenged claims. Therefore, NAD did not review the claims on their merits and will treat the discontinued claims, for compliance purposes, as though NAD recommended they be discontinued.
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. Per NAD/NARB Procedures, this release may not be used for promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than 20 globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create fair competition for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and create fair competition for business.
Contact Information
Name: Jennifer Rosenberg
Email: press@bbbnp.org
Job Title: Media Relations
LexisNexis Enhances Lexis+ with Protégé Platform by Integrating Anthropic Cowork Legal Plugin
NEW YORK, FEBRUARY 24, 2026 — LexisNexis® Legal & Professional today announced the integration of Anthropic’s legal plugin (“Legal Plugin”) into the Lexis+® with Protégé™ platform (“Protégé”). The company has been evaluating the Legal Plugin capabilities since before its market release. This integration enhances hundreds of existing AI and agentic AI legal workflow capabilities available via Protégé and is part of the company’s process to continuously evaluate and incorporate new technologies or capabilities that help customers achieve better outcomes in trusted LexisNexis solutions.
The integration enables Protégé users to automate finished, verifiable legal work product in multiple ready-to-use formats with their work grounded accurately in the company’s vast 200-billion document repository – with four million new documents added daily – of essential, unique, constantly updated, Shepardized, and linked legal content. The integration leverages the Legal Plugin all within the Protégé world-class, private, secure, and trusted technology environment.
The Legal Plugin integration into Protégé has been tested over the last few weeks by a limited group of customers in commercial preview as part of the company’s process to rapidly evaluate and incorporate new technologies and capabilities across multiple models and plugins that add value for legal customers and their daily work.
Commenting on the Legal Plugin integration into Protégé, Nancy Kuhn, partner, Shulman Rogers, said, “I appreciate that Protégé automatically validates the legal citations. That feature is a huge timesaver. The end product, after verification and editing, is also easy to format in Word so that it can be quickly finalized…With so many AI tools out there, it’s helpful that Protégé minimizes the number of choices by integrating these experiences into one solution.”
In a product testing forum last week, an AmLaw 100 third-year associate noted that the new Legal Plugin embedded in Protégé “will take me from prompt to finished work product much faster and better visually than I can do it. It’s like going from driving a horse and buggy to driving a Maserati. I could not have imagined it being this powerful.”
“LexisNexis is delighted to integrate the Legal Plugin into Protégé to further automate authoritative legal workflows and deliver more value to customers,” said Sean Fitzpatrick, CEO Global Legal, LexisNexis Legal & Professional. “We are excited to put this in customers’ hands, enabling new, interactive, and intuitive ways to generate ready-to-use, fully formatted legal work that we believe customers will not want to be without.”
Within Protégé, the integrated Legal Plugin enables legal professionals to:
- Automatically complete tasks like ‘check a data protection agreement’ against the LexisNexis repository of up-to-date, authoritative compliance regulations; ‘check a contract’ against a LexisNexis checklist; or ‘generate a research-style briefing’ on a specific legal question or topic.
- Synchronize outputs across multiple documents, presentations, and spreadsheets to create a unified, formatted, and branded set of legal materials. Users can create final, fully formatted Word documents, coupled with high-level client presentations and detailed spreadsheets, which have received very favorable customer feedback.
- Soon, users will be able to accomplish even more. For example, LexisNexis is developing the ability for a user to enter “I want to generate a 50-state survey” in Protégé’s single conversational prompt box. From this single prompt, legal professionals can automatically generate a coordinated set of deliverables, including a polished Word memo, a client-ready presentation, and a tracking spreadsheet that requires minimal editing and remains consistent across formats.
Protégé will continue to integrate additional Anthropic skills as they are released to the market within the platform’s easy-to-use prompt box.
LexisNexis is providing increasing support for customers in using and adopting Protégé including the Legal Plugin via a white glove service that helps organizations unlock the full value of these products with expert guidance and practical support. Specialized teams help customers build custom workflows, migrate existing workflows, standardize workflows across organizations, and provide team training and onboarding.
Protégé brings together an advanced AI infrastructure developed specifically for legal work and the world’s most comprehensive collection of citable legal authority to help professionals complete higher-quality legal work faster while maintaining the rigor and control required for legal practice. This commercial preview reflects LexisNexis’ continued leadership in authoritative legal AI innovation.
Following the commercial preview, broad availability will be guided by customer feedback and development.
To learn more about Protégé: www.lexisnexis.com/protege and Lexis+ with Protégé: www.lexisnexis.com/ai.
About LexisNexis Legal & Professional
LexisNexis Legal & Professional provides AI-powered legal, regulatory, business information, analytics, and workflows that help customers increase their productivity, improve decision-making, achieve better outcomes, and advance the rule of law around the world. As a digital pioneer, the company was the first to bring legal and business information online with its Lexis® and Nexis® services. LexisNexis Legal & Professional, which serves customers in more than 150 countries with 11,900 employees worldwide, is part of RELX, a global provider of information-based analytics and decision tools for professional and business customers.
###
Contact Information
Name: Anuj Baveja
Email: anuj.baveja@lexisnexis.com
Job Title: Director of Communications – North America & UK
General Availability of Lexis+ with Protégé Sets New Standard for Automating Legal Work with Easy-to-Use, Authoritative AI Workflows
Grounded in citable authority, Lexis+ with Protégé delivers the most integrated and intuitive legal AI platform to support complex legal work, custom workflow design, and broader AI tasks
NEW YORK, FEBRUARY 24, 2026 — LexisNexis® Legal & Professional, a global leader in information, analytics, and AI-powered legal workflow solutions, today announced the general availability of Lexis+® with Protégé , delivering purpose-built, end-to-end legal AI workflows with a new user interface designed to make trusted legal work possible with one prompt. Building on the authoritative agentic AI capabilities available via Lexis+ AI – including conversational research, personalized legal drafting, document upload, summarization, and analysis – legal professionals can now automate their work to an even greater extent using Lexis+ with Protégé, our new, integrated flagship platform that replaces Lexis+ AI. Customers can ask any legal question using a single, conversational prompt box, and seamlessly access pre-built and custom workflow capabilities. As with Lexis+ AI before it, Lexis+ with Protégé is underpinned by Shepard’s® citations and lets users securely work with their own documents, trusted LexisNexis content, and leading general AI models to achieve better legal outcomes in an integrated platform that enables greater ease-of-use and less risk from switching between multiple AI tools.
, delivering purpose-built, end-to-end legal AI workflows with a new user interface designed to make trusted legal work possible with one prompt. Building on the authoritative agentic AI capabilities available via Lexis+ AI – including conversational research, personalized legal drafting, document upload, summarization, and analysis – legal professionals can now automate their work to an even greater extent using Lexis+ with Protégé, our new, integrated flagship platform that replaces Lexis+ AI. Customers can ask any legal question using a single, conversational prompt box, and seamlessly access pre-built and custom workflow capabilities. As with Lexis+ AI before it, Lexis+ with Protégé is underpinned by Shepard’s® citations and lets users securely work with their own documents, trusted LexisNexis content, and leading general AI models to achieve better legal outcomes in an integrated platform that enables greater ease-of-use and less risk from switching between multiple AI tools.
“Legal professionals are increasingly seeking integrated legal AI work environments,” said Sean Fitzpatrick, CEO Global Legal, LexisNexis Legal & Professional. “Lexis+ with Protégé brings together the most comprehensive legal content, verifiable and citable sources, trusted legal workflows, and access to leading general AI models in a single, secure workspace. With a purpose-built AI infrastructure designed for legal practice, Protégé enables teams to complete their legal work end-to-end with confidence that outputs are validated and consistent wherever they work.”
New workflow capabilities within Lexis+ with Protégé automate drafting, review, analysis, and citation checking into scalable, repeatable legal processes that simplify complex legal work and deliver consistent, high-quality results across teams. At general availability, Lexis+ with Protégé supports a broad range of ready-to-run and customizable legal workflows grounded in world-leading LexisNexis authoritative content and legal knowledge graph – and 200 billion documents with more than 4 million new documents added daily – and citation, trust, and validity signals continuously updated, delivering depth, currency, and connectivity that cannot be replicated. Within the new platform, legal professionals can streamline and automate high-quality work by using:
- Pre-built, configurable workflows that can be run as-is or adapted to firm and department standards, including litigation workflows, transactional workflows, and everyday legal workflows for broader AI tasks via LexisNexis-enhanced general AI models from Anthropic, Google, and OpenAI. Examples include drafting a motion to dismiss; generating full discovery and deposition documents; creating transactional documents or clauses; redlining agreements against internal standards; analyzing key provisions and identifying high-risk clauses; comparing similar arguments or laws; and summarizing interviews.
- A no-code Custom Workflow Builder that allows law firms and corporate legal departments to design, test, and share multi-step workflows tailored to their own standards and playbooks, turning institutional knowledge into repeatable legal processes that can be used consistently across teams.
Coming soon to Lexis+ with Protégé:
- Advanced practice-area workflows that extend this foundation with domain-specific workflows for high-value matters such as civil litigation, mergers and acquisitions, real estate, and labor and employment.
- New agentic and persona workflows, building on the agentic workflows LexisNexis announced in 2024, to further advance how legal professionals interact with artificial intelligence.
Additionally, the company is rolling out a new white glove service that helps organizations unlock the full value of workflows through a high-touch program that combines expert guidance with practical support. Specialized workflow teams help customers build custom workflows, migrate existing workflows, and standardize workflows across organizations, and provide team training and onboarding.
Following US general availability, Lexis+ with Protégé begins rolling out across global markets throughout 2026.
To learn more about Protégé: www.lexisnexis.com/protege and Lexis+ with Protégé: www.lexisnexis.com/ai.
About LexisNexis Legal & Professional
LexisNexis Legal & Professional provides AI-powered legal, regulatory, business information, analytics, and workflows that help customers increase their productivity, improve decision-making, achieve better outcomes, and advance the rule of law around the world. As a digital pioneer, the company was the first to bring legal and business information online with its Lexis® and Nexis® services. LexisNexis Legal & Professional, which serves customers in more than 150 countries with 11,900 employees worldwide, is part of RELX, a global provider of information-based analytics and decision tools for professional and business customers.
###
Contact Information
Name: Anuj Baveja
Email: anuj.baveja@lexisnexis.com
Job Title: Director of Communications – North America & UK
National Advertising Division Recommends PrettyBoy Modify or Discontinue Certain Skincare Claims

New York, NY – February 19, 2026 – Following a review of advertising for PrettyBoy Skincare, BBB National Programs’ National Advertising Division recommended that PrettyBoy, Inc. modify or discontinue certain claims, including ratings on third-party apps and before-and-after photographs.
PrettyBoy markets skincare products directed to men. The National Advertising Division (NAD) opened this inquiry based on its concerns relating to the Yuka App superlative health ranking and star ratings for PrettyBoy. In addition, NAD examined a number of performance claims.
“100/100 Health Score (via the Yuka App)”
The Yuka mobile app scans and analyzes product labels and assigns a score based on an independent assessment of ingredients and their impact on human health or the environment. Yuka rated PrettyBoy’s Revival Recovery Gel Moisturizer “100/100” because it does not contain “harmful parabens” or a “harmful UV filter,” and the app lists the product’s ingredients as “risk-free.”
NAD determined that the PrettyBoy webpage where the claim appears does not make the basis of the score clear and recommended that PrettyBoy modify its advertising to clarify the basis of the ranking.
Before and After Photographs
NAD noted that before-and-after photographs that appear in PrettyBoy’s advertising constitute product performance claims and must be supported by evidence representative of what consumers can expect when using the product.
The PrettyBoy photos depict reductions in redness associated with eczema, and reductions in fine lines and undereye bags. NAD determined that these objectively provable improvements require support, and claims related to eczema require competent and reliable scientific evidence as support.
NAD found that PrettyBoy’s reliance on the National Eczema Association’s Seal of Acceptance for two of its products, without any underlying testing, was not sufficient to support the depictions and recommended that the before-and-after photographs be discontinued.
During the inquiry, PrettyBoy permanently discontinued the claim “Trusted by 20,000 Men (5-star rating).” Therefore, NAD did not review the claim on its merits and will treat the discontinued claim, for compliance purposes, as though NAD recommended it be discontinued and PrettyBoy agreed to comply.
In its advertiser statement, PrettyBoy stated that it “appreciates NAD’s review and will comply with NAD’s recommendation.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. Per NAD/NARB Procedures, this release may not be used for promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than 20 globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create fair competition for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and promoting fair competition for business.
Contact Information
Name: Jennifer Rosenberg
Email: press@bbbnp.org
Job Title: Media Relations
National Advertising Division Recommends T-Mobile Modify or Discontinue In-Flight Wi-Fi Claims

New York, NY – February 12, 2026 – In a Fast-Track SWIFT challenge brought by Verizon Communications Inc., BBB National Programs’ National Advertising Division recommended that T-Mobile US, Inc. discontinue or modify advertising claims concerning the cost of a free in-flight Wi-Fi benefit offered by T-Mobile.
At issue for the National Advertising Division (NAD) was an express claim on T-Mobile’s website stating “T-Mobile: In-flight Wi-Fi – Included; Verizon: In-flight Wi-Fi – $ 147.00/mo.” and the implied claim that Verizon customers incur $147 in monthly in-flight Wi-Fi costs, while the same service is included with T-Mobile plans.
NAD found that the challenged in-flight Wi-Fi advertising did not accurately communicate the benefit for T-Mobile customers or the cost Verizon customers would incur. The benefit for T-Mobile customers is that they have unlimited access to full-flight texting and free Wi-Fi on certain airlines through their T-Mobile plan. T-Mobile’s disclosures do not indicate which major airlines are covered by the benefit. Verizon customers do not receive such a benefit through their plan, although Verizon customers may have in-flight Wi-Fi from other sources.
NAD determined that presenting “In-flight Wi-Fi – $147.00/mo” under the Verizon column in T-Mobile’s savings calculator could convey that Verizon charges for in-flight Wi-Fi or that Verizon customers typically incur high charges for such benefit. While T-Mobile stated that it intended to communicate only what consumers would have to pay to get the comparable Wi-Fi benefit that is included in the T-Mobile plan, the manner in which the in-flight Wi-Fi benefit is presented goes beyond that limited message. T-Mobile’s explanation of the benefit is ambiguous and inadequate, especially in the context of its broader savings claims.
While T-Mobile submitted evidence showing that its customers frequently use the free in-flight Wi-Fi benefit, NAD found that T-Mobile did not submit evidence to support claims regarding what Verizon customers pay. NAD concluded that T-Mobile did not meet its burden to provide a reasonable basis for the challenged claims.
Accordingly, NAD recommended T-Mobile discontinue the challenged in-flight Wi-Fi claims or modify them to clearly and conspicuously disclose the nature of its in-flight Wi-Fi benefit by communicating that the fees that T-Mobile customers can potentially avoid with their plans are those charged by certain airlines and to avoid communicating that non-T-Mobile customers typically pay the monthly cost of in-flight Wi-Fi set forth by T-Mobile in its advertising.
In its advertiser statement, T-Mobile stated that it “will comply with NAD’s recommendation with respect to its already discontinued advertising claim.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. Per NAD/NARB Procedures, this release may not be used for promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than 20 globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create fair competition for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and promoting fair competition for business.
Contact Information
Name: Jennifer Rosenberg
Email: press@bbbnp.org
Job Title: Media Relations
