
New York, NY – February 26, 2025 – In a Fast-Track SWIFT challenge brought by Animal Partisan, BBB National Programs’ National Advertising Division found that Certified Angus Beef‘s claims that the Beef Quality Assurance (BQA) program represents “best practices” and “highest standards” are supported.
Animal Partisan is a nonprofit organization whose stated mission is improving animal welfare and combating the suffering of animals in agriculture. Certified Angus Beef, also a nonprofit organization, owns the Certified Angus Beef® logo and promotes the BQA program to beef producers.
Fast-Track SWIFT is an expedited challenge process designed for single-issue advertising cases brought to the National Advertising Division (NAD). At issue is whether claims on Certified Angus Beef’s website, social media, and in marketing materials are unsupported given Animal Partisan’s claims that other standards for cattle care, such as those set by Humane Farm Animal Care (HFAC) and Global Animal Partnership (GAP), are considered more rigorous.
Certified Angus Beef promotes the BQA program as ensuring “best practices in animal handling, animal care, and responsible antibiotic use.” The organization claims BQA provides educational resources, supports humane care practices, and helps farmers maintain high standards and stay updated with animal welfare practices.
NAD determined that Certified Angus Beef’s references to “best practices” and “highest standards” in the context of the challenged advertising do not convey the message that BQA represents the most superior level of care available for cattle in the beef industry and does not convey a message of objective superiority over all other standards. Rather, “best practices” generally refer to techniques developed through a structured process to represent a trusted standard. The advertising conveys that BQA is a high standard recognized by the industry.
Therefore, NAD determined that the challenged claims are substantiated as they appear in the context of the challenged advertising.
In its advertiser statement, Certified Angus Beef stated it is “pleased that the NAD recognized the high standards for cattle care set by the program.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. Per NAD/NARB procedures, this release may not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and leveling the playing field for business.
Contact Information
Name: Jennie Rosenberg
Email: jrosenberg@bbbnp.org
Job Title: Media Relations
In National Advertising Division Challenge, Kevin Hart Modifies Social Media Posts to Disclose Material Connections to JPMC and Fabletics

New York, NY – February 20, 2025 – As part of its routine monitoring program, BBB National Programs’ National Advertising Division inquired about celebrity influencer Kevin Hart’s relationship with JPMorgan Chase Bank, N.A. (JPMC) and Fabletics, Inc. As a result of its inquiry, Kevin Hart modified his social media posts to include clear and conspicuous disclosures of his material connections to the brands.
Hart, an investor in Fabletics Men and a paid endorser for JPMC, has 177 million Instagram followers, where he posts about the brands he supports.
At issue for the National Advertising Division (NAD) was whether Kevin Hart’s social media posts disclosed his material connections with JPMC and Fabletics, focusing on the sufficiency and placement of disclosures on his Instagram profile.
Although Kevin Hart stated that his followers are likely aware of his connections due to his long-standing endorsements, NAD found that a significant minority of the audience might not be aware of Hart’s affiliations due to varying levels of engagement with athletic brands or celebrity endorsements.
Therefore, NAD recommended a clear and conspicuous disclosure of Hart’s relationship with JPMC and Fabletics.
In response to NAD’s inquiry, Hart’s team advised NAD that the posts have been updated to include disclosures of these material connections and noted that Hart and the team are committed to complying with the Federal Trade Commission’s Endorsement Guides.
In its advertiser statement, Kevin Hart said that he will comply with NAD’s decision.
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. This press release shall not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. The National Advertising Division reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and leveling the playing field for business.
Contact Information
Name: Jennie Rosenberg
Email: jrosenberg@bbbnp.org
Job Title: Media Relations
Lawyers impatient for firms to invest in technology and innovation
Clients need their firms to be fast, cost-effective and agile. However, 70% of private practice lawyers said their firm is adequate, slow or very slow at implementing new technology while 67% said the same about responding to change.
LONDON, 20 February 2025 – Today, LexisNexis® Legal & Professional, a leading global provider of AI-powered analytics and decision tools, released a new report – Innovating the client experience: Law firms can offer much more than legal expertise – which reveals a sharp demand from lawyers for greater tech investment.
The survey of 800+ UK legal professionals found in-house legal counsel will need their external counsel to be cost-effective (74%), responsive and agile (67%), and offer specialist legal expertise (44%), all of which require an investment in new technology and innovation.
However, the survey revealed only 18% of private practice lawyers believe their firm is fast or very fast at implementing new technology. Nearing three-quarters (70%) said their firm is adequate, slow or very slow. Responding to change and making use of data and analytics are also areas in need of greater innovation. Two-thirds (67%) of legal professionals said their firm is adequate, slow or very slow at responding to change, while more than half (58%) said the same about data and analytics.
The survey also revealed many firms are being slowed down by sluggish systems and processes. When asked how quickly their firm conducts legal research, more than half (52%) of private practice lawyers rated their firm as adequate, slow or very slow. Drafting and reviewing legal documents was also considered a challenging area by many, with 45% saying their firm is adequate, slow or very slow. Concerningly, more than a third (35%) said their firm is adequate, slow or very slow at delivering legal work in general. As a potential solution, more than half (57%) of private practice lawyers expect their firms to become more reliant on AI for legal research and document review in the next one to three years, which could streamline processes.
Another potential downside to failing to invest in innovation is a loss of talent. If a firm failed to embrace AI, a quarter (25%) of all lawyers said it would negatively impact their careers, and 11% said they would consider leaving. This escalated at larger firms, with one third (36%) of lawyers saying it would negatively impact their career, and one in five (19%) saying they would consider leaving.
Stuart Greenhill, Senior Director of Segments at LexisNexis UK, commented “To remain competitive, firms will need to deliver a superior, data-driven legal service, at the same cost or lower, and at pace – and to keep clients informed of any legal or regulatory developments.
Achieving all this without the help of modern technology will be difficult. To secure client relationships, firms will need to invest in a streamlined, data-driven client offering.”
Notes for editors
- This press release is embargoed until 00:01 GMT on Thursday 20th February 2025
- The report can be accessed here: www.lexisnexis.co.uk/research-and-reports/innovation-as-a-competitive-edge.html
Contact Information
Name: Matthew Leopold
Email: matthew.leopold@lexisnexis.co.uk
Job Title: Head of Communications
National Advertising Review Board Recommends GuruNanda Discontinue Pulling Oil Teeth Whitening Claims

New York, NY – February 18, 2025 – A panel of the National Advertising Review Board (NARB), the appellate advertising body of BBB National Programs, recommended that GuruNanda, LLC discontinue “natural teeth whitening” claims for its pulling oil product.
GuruNanda’s pulling oil, a mouthwash-type product, is based on a traditional Ayurveda practice that involves swishing oils, such as coconut, sesame, and sunflower oil, between the teeth to promote oral health.
The advertising at issue had been challenged by The Procter & Gamble Company before BBB National Programs’ National Advertising Division (NAD). Following NAD’s decision (Case No. 7377), GuruNanda appealed NAD’s recommendation to discontinue the “natural teeth whitening” claim.
The NARB panel determined that NAD’s decision should be affirmed because the two clinical studies submitted by GuruNanda in support of its claim were not sufficiently rigorous to meet the standard of competent and reliable scientific evidence applicable here.
The NARB panel further noted that while the results in these exploratory studies may well point to a possible whitening effect for swishing oil, the research to date does not satisfy the standards for scientific claim support.
Therefore, the NARB panel recommended that GuruNanda discontinue the claim “natural teeth whitening.”
In its advertiser statement, GuruNanda stated that though it “strongly disagrees with NARB’s analysis,” it will “comply with NARB’s recommendation.” GuruNanda further stated that “additional scientific studies were already underway…that will continue to demonstrate” the product’s whitening abilities.
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. Per NAD/NARB procedures, this release may not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Review Board (NARB): The National Advertising Review Board (NARB) is the appellate body for BBB National Programs’ advertising self-regulatory programs. NARB’s panel members include 85 distinguished volunteer professionals from the national advertising industry, agencies, and public members, such as academics and former members of the public sector. NARB serves as a layer of independent industry peer review that helps engender trust and compliance in NAD, CARU, and DSSRC matters.
Contact Information
Name: Jennie Rosenberg
Email: jrosenberg@bbbnp.org
Job Title: Media Relations
LexisNexis Announces “Top 100 Global Innovators” for 2025, Recognizing Leading Companies Driving Innovation in the Global Economy
Top 100 Global Innovators Identified by LexisNexis Intellectual Property Solutions Across Database of ~16 Million Patent Families Using Patent Asset Index Methodology
New York, NY – February 18, 2025 – LexisNexis® Legal & Professional today announced the “Top 100 Global Innovators” for 2025, the prestigious roster of companies around the world that are driving innovation in the global economy, based on exceptional shifts in patent portfolio quality over the last two years. The top 100 companies were identified through an analysis by LexisNexis® Intellectual Property Solutions across a database of nearly 16 million global patent families using its proven Patent Asset Index methodology.
“Measuring and comparing innovation across different industries, technologies, and geographies may seem an impossible task, but the LexisNexis® Patent Asset Index provides a trusted methodology to identify 100 companies that are leading innovation globally,” said Marco Richter, Senior Director of IP Analytics and Strategy for LexisNexis Intellectual Property Solutions. “From trillion-dollar giants to start-ups whose entire team could fit in a movie theater, this year’s Top 100 Global Innovators span the spectrum, but they share a common commitment to pushing the frontiers of research and advancing human knowledge. We commend all of this year’s honorees on their leadership in creating a better world, and we look forward to watching their continued innovation in the future.”
Top 100 Global Innovators by Industry Sector
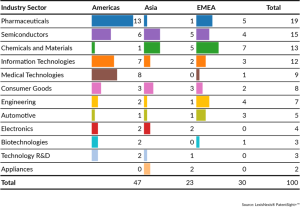
For the fourth consecutive year, the U.S. dominated the Top 100 Global Innovators list with 47 companies, including the majority recognized in industries like Pharmaceuticals, Information Technologies, and Medical Technologies. Other countries with notable number of Top 100 innovators include Germany (12), China (7), Japan (5), and Korea (7).
There were 18 new entrants among this year’s Top 100 honorees, including Chengdu Qinchuan IoT, Coupang, CXMT, Henkel, International Flavors & Fragrances, JFE Holdings, Kokusai Electric, L’Oréal, Novocure, Novonesis, RTX Corp, Siemens, SK Innovation, Topsoe, Wave Life Sciences, Willow Laboratories, ZF, and ZTE. Five of the new entrants are US-based, while three new entrants each are from Germany and China.
The Top 100 Global Innovators list was released as part of the Innovation Momentum 2025: The Global Top 100 report. The report also focused on the blurring boundaries between traditional stand-alone industries and technologies as innovations like digitization, wireless connectivity, and mobile devices have started to erase boundaries that once seemed clear. The report illustrated the growing overlap in areas like autonomous and electric vehicles, 5G technologies, and biotechnology/pharma.
The report also explored the contribution of AI-related patents to the total portfolio strength, as measured by the Patent Asset Index, of Top 100 companies in the four largest industries represented in the list. Unsurprisingly, the Information Technologies industry stands at the forefront of AI innovation with roughly 15% of the total strength of the aggregate portfolios of companies represented in the industry derived from AI-related inventions.
The following are this year’s Top 100 Global Innovators:
Top 100 Global Innovators in Alphabetical Order with
Global Headquarters and Industry Sector
| Patent Owner | HǪ | Industry |
| 10x Genomics | US | Biotechnologies |
| Acuitas Therapeutics | CA | Pharmaceuticals |
| Align Technology | US | Medical Technologies |
| Alnylam Pharmaceuticals | US | Pharmaceuticals |
| Alphabet | US | Information Technologies |
| Amazon | US | Information Technologies |
| Amgen | US | Pharmaceuticals |
| Apple | US | Electronics |
| Applied Materials | US | Semiconductors |
| ARAMCO | SA | Chemicals and Materials |
| Arvinas | US | Pharmaceuticals |
| ASM | NL | Semiconductors |
| ASML | NL | Semiconductors |
| AutoStore | NO | Information Technologies |
| BASF | DE | Chemicals and Materials |
| Becton, Dickinson | US | Medical Technologies |
| BioNTech | DE | Pharmaceuticals |
| Bosch | DE | Automotive |
| Bristol-Myers Squibb | US | Pharmaceuticals |
| British American Tobacco | GB | Consumer Goods |
| CATL | CN | Chemicals and Materials |
| Chengdu Ǫinchuan IoT | CN | Technology R&D |
| Coupang | US | Information Technologies |
| CureVac | DE | Pharmaceuticals |
| CXMT | CN | Semiconductors |
| Daikin | JP | Appliances |
| Deere & Co | US | Engineering |
| DSM-Firmenich | CH | Chemicals and Materials |
| Edwards Lifesciences | US | Medical Technologies |
| Eli Lilly | US | Pharmaceuticals |
| Ericsson | SE | Information Technologies |
| Gilead Sciences | US | Pharmaceuticals |
| Henkel | DE | Chemicals and Materials |
| Huawei | CN | Information Technologies |
| Hyundai Motor | KR | Automotive |
| IBM | US | Information Technologies |
| Illumina | US | Biotechnologies |
| Incyte | US | Pharmaceuticals |
| Infineon | DE | Semiconductors |
| International Flavors & Fragrances | US | Chemicals and Materials |
| Intel | US | Semiconductors |
| InterDigital | US | Technology R&D |
| Intuitive Surgical | US | Medical Technologies |
| Japan Tobacco | JP | Consumer Goods |
| JFE Holdings | JP | Engineering |
| Johnson & Johnson | US | Pharmaceuticals |
| Juniper Networks | US | Information Technologies |
| KLA | US | Semiconductors |
| Kokusai Electric | JP | Semiconductors |
| KT&G | KR | Consumer Goods |
| L’Oréal | FR | Consumer Goods |
| Lam Research | US | Semiconductors |
| LG Chem | KR | Chemicals and Materials |
| LG Electronics | KR | Electronics |
| Magic Leap | US | Electronics |
| Masimo | US | Medical Technologies |
| MediaTek | TW | Semiconductors |
| Medtronic | IE | Medical Technologies |
| Meta | US | Information Technologies |
| Moderna Therapeutics | US | Pharmaceuticals |
| Nike | US | Consumer Goods |
| Novartis | CH | Pharmaceuticals |
| Novocure | CH | Pharmaceuticals |
| Novonesis | DK | Biotechnologies |
| Nvidia | US | Semiconductors |
| Ocado | GB | Information Technologies |
| Ofinno | US | Technology R&D |
| OMV | AT | Chemicals and Materials |
| Procter & Gamble | US | Consumer Goods |
| Pfizer | US | Pharmaceuticals |
| Philip Morris | US | Consumer Goods |
| Ǫualcomm | US | Semiconductors |
| Regeneron | US | Pharmaceuticals |
| ResMed | US | Medical Technologies |
| Revolution Medicines | US | Pharmaceuticals |
| Rolls-Royce | GB | Engineering |
| RTX Corp | US | Engineering |
| Saint-Gobain | FR | Chemicals and Materials |
| Samsung | KR | Electronics |
| Samsung SDI | KR | Chemicals and Materials |
| Sanofi | FR | Pharmaceuticals |
| Siemens | DE | Engineering |
| SK Innovation | KR | Chemicals and Materials |
| Smoore | CN | Consumer Goods |
| Snap | US | Information Technologies |
| Stryker | US | Medical Technologies |
| Techtronic | HK | Appliances |
| Tesla | US | Automotive |
| thyssenkrupp | DE | Engineering |
| Tokyo Electron | JP | Semiconductors |
| Topsoe | DK | Chemicals and Materials |
| TRUMPF | DE | Engineering |
| TSMC | TW | Semiconductors |
| VW Group | DE | Automotive |
| Wave Life Sciences | SG | Pharmaceuticals |
| Willow Laboratories | US | Medical Technologies |
| Yantai Jereh Oilfield Services | CN | Chemicals and Materials |
| ZEISS | DE | Semiconductors |
| ZF | DE | Automotive |
| ZTE | CN | Information Technologies |
While the report focused on corporate patent owners, a parallel analysis was done of academic and public research innovators to recognize their achievements as well. Among those institutions, Broad Institute, Fraunhofer, Harvard, MIT, Stanford University, University of California, and Zhejiang University were recognized for demonstrating exceptional Innovation Momentum, while developing relevant and high-quality patent portfolios.
The full report is available for download at https://www.lexisnexisip.com/innovation-momentum-report.
About the Methodology
LexisNexis® conducted an analysis across its database of nearly 16 million global patent families to identify companies that are leaders in global innovation. The methodology was based on the LexisNexis Patent Asset Index, which was used to measure the strength and quality of a company’s patent assets. The analysis tracked exceptional shifts in patent portfolio quality over the last two years to identify the companies named as the “Top 100 Global Innovators” in this year’s “Innovation Momentum 2025” report.
About LexisNexis® Legal & Professional
LexisNexis® Legal & Professional provides legal, regulatory, and business information and analytics that help customers increase their productivity, improve decision-making, achieve better outcomes, and advance the rule of law around the world. As a digital pioneer, the company was the first to bring legal and business information online with its Lexis® and Nexis® services. LexisNexis Legal & Professional, which serves customers in more than 150 countries with 11,800 employees worldwide, is part of RELX, a global provider of information-based analytics and decision tools for professional and business customers.
About LexisNexis® Intellectual Property Solutions
LexisNexis® Intellectual Property Solutions bring clarity to innovation for businesses worldwide. We enable innovators to accomplish more by helping them make informed decisions, be more productive, comply with regulations, and ultimately achieve a competitive advantage for their business. Our broad suite of workflow and analytics solutions (LexisNexis® PatentSight+ , LexisNexis® Classification, LexisNexis® TechDiscovery, LexisNexis® IPlytics
, LexisNexis® Classification, LexisNexis® TechDiscovery, LexisNexis® IPlytics , LexisNexis PatentOptimizer®, LexisNexis PatentAdvisor®, and LexisNexis TotalPatent One®, LexisNexis® IP DataDirect), enables companies to be more efficient and effective at bringing meaningful innovations to our world. We are proud to directly support and serve these innovators in their endeavors to better humankind.
, LexisNexis PatentOptimizer®, LexisNexis PatentAdvisor®, and LexisNexis TotalPatent One®, LexisNexis® IP DataDirect), enables companies to be more efficient and effective at bringing meaningful innovations to our world. We are proud to directly support and serve these innovators in their endeavors to better humankind.
LexisNexis | Intellectual Property Solutions
Bringing clarity to innovation
Contact Information
Name: Andrew Weinstein
Email: Andrew.Weinstein@LexisNexis.com
Job Title: PR Consultant
New BFGoodrich g-Force Phenom T/A tire helps drivers own the road


- Ultra high performance summer tire joins the g-Force Family
- g-Force Phenom T/A tire engineered to excel on both wet, dry pavement
- Phased launch begins in U.S. with 20 sizes available Feb. 14
GREENVILLE, S.C., Feb. 13, 2025 – BFGoodrich Tires has unleashed the g-Force Phenom T/A tire: an ultra high performance summer tire that looks and plays the part by delivering excellent performance in both wet and dry conditions.

“We’re thrilled to launch the g-Force Phenom T/A tire, which was built to be the easiest upgrade enthusiasts can make to boost the performance and look of their cars,” said Andrew Besancon, Senior Director of Recreational Brands. “BFGoodrich has been engineering category-defining tires for more than 150 years. With this new tire, we’re offering consumers strong performance and a smart value from a race-proven brand.”
The g-Force Phenom T/A tire provides the following features and benefits:
Designed to corner: Stiff sidewalls, shallow shoulder blocks and offset shoulder grooves are designed to provide better feedback at the limit and enhance cornering stability.1
Engineered for grip: A dual-zone tread design features an optimized wet zone for water evacuation and a dry zone for maximum road contact for ultimate confidence and control.2
Built for braking: Its new summer silica-infused compound is tuned for ultimate performance and delivers wet grip, outperforming leading competitors.
- The BFGoodrich g-Force Phenom T/A tire has better wet braking distance than the Yokohama ADVAN Apex V601 tire and other leading competitors.3
Sizes and availability
BFGoodrich will roll out 50 sizes during the next two months, with 20 sizes available Feb. 14 and 30 additional sizes coming in April. The phased launch will continue with additional sizes later in 2025 and into spring 2026.

To find your size, availability and pricing, visit bfgoodrichtires.com or your local BFGoodrich dealer.
The g-Force Phenom T/A tire, the newest entry in BFGoodrich’s g-Force Family of tires, carries a 60-day consumer satisfaction guarantee.
Skip Barber Racing School’s tire choice
The g-Force Phenom T/A tire is the official tire of the Skip Barber Racing School. Stay tuned for collaboration between the world’s largest racing school and BFGoodrich.
For now, here’s a sneak peek!
1 Based on internal Braking Distance, Autocross in Wet Handling, and Dry Analytical Adherence results using a 2019 Genesis G70 vehicle with BFGoodrich g-Force Sport Comp-2 tire in size 245/40ZR18 93W versus BFGoodrich g-Force Phenom T/A tire in tire size 245/40R18 XL 97Y. Actual on-road results may vary.
2 Based on internal Braking Distance, Autocross in Wet Handling, Max Handling, and Dry Analytical Adherence results using a 2019 Genesis G70 vehicle with BFGoodrich g-Force Sport Comp-2 tire in tire size 245/40ZR18 93W versus BFGoodrich g-Force Phenom T/A tire in tire size 245/40R18 XL 97Y. Actual on-road results may vary.
3 Based on internal wet braking tests at 50 mph comparing the BFGoodrich g-Force Phenom T/A tire in tire size 245/40ZR18 XL 97W versus the Yokohama ADVAN Apex V601 tire in tire size 245/40ZR18 XL 97Y, the Falken Azenis FK 510 tire in tire size 245/40ZR18 97Y, the Firestone Firehawk Indy 500 tire in tire size 245/40R18 XL 97W, and the Hankook Ventus V12 EV02 tire in tire size 245/40ZR18 XL 97Y, using a 2019 Genesis G70 vehicle. Actual on-road results may vary.
About BFGoodrich Tires
BFGoodrich Tires is dedicated to providing high performance tires for those who have a passion for driving in virtually any environment. Combining technical expertise with 50 years of motorsports experience, BFGoodrich delivers tires for a full range of driving experiences from ultra-high-performance street to off-road terrain with one common theme – extreme performance. Come upgrade your performance with BFGoodrich and see where our tires can take you at BFGoodrichTires.com and BFGoodrichRacing.com, as well as on Facebook and Instagram at @BFGoodrichTires and TikTok at @BFGoodrich.
Media contact:
Andrew Festa
BFGoodrich Tires | andrew.festa@michelin.com
National Advertising Division Recommends Total Wireless Modify Monthly Pricing and Free Phone Offer Claims

New York, NY – February 13, 2025 – National Programs’ National Advertising Division recommended that Total Wireless, a brand of Tracfone Wireless, a subsidiary of Verizon Communications Inc., modify its ads to avoid suggesting customers get both four lines for $25/month and four free 5G phones.
Fast-Track SWIFT is an expedited challenge process designed for single-issue advertising cases brought to the National Advertising Division (NAD). At issue was whether Total Wireless made an unsupported claim that its customers can get both four lines of wireless service for $25 per month and four free 5G phones.
Total Wireless aired a commercial claiming it “has your back every day,” unlike other prepaid brands such as Metro by T-Mobile. The spokesman says, “Fact them,” with screen text restating the qualifying conditions for each offer: “We run on the network with America’s fastest 5G speeds for as low as $25 a month. They don’t,” that Total Wireless offers a five-year price guarantee, as well as “and to top it all off, we’ll give you up to four free 5G phones,” with text on screen that qualifies the four free phones is applicable “When you switch to the Total 5G+ Unlimited plan.”
After review, NAD found that though the visual cues in the commercial delineate the offers, the audio does not.
In addition, when presenting the last offer, NAD determined that the phrase, “and to top it all off, we’ll give you up to four free 5G phones,” implies the last part of a single offer. However, the audio does not indicate that Total Wireless offers different plan tiers and that the available benefits may depend on the particular plan that the consumer selects.
Therefore, NAD determined that the commercial conveys the unsupported message that Total Wireless customers can get both four lines of wireless service for $25 per month and get four free 5G phones regardless of the plan they select, and recommended Total Wireless modify its advertising to avoid conveying such a message.
In its advertiser statement Total Wireless stated that while it, “disagrees with the decision, it supports industry self-regulation and will comply with NAD’s recommendations in its future advertising.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Division: The National Advertising Division (NAD) of BBB National Programs provides independent self-regulation and dispute resolution services, guiding the truthfulness of advertising across the U.S. NAD reviews national advertising in all media and its decisions set consistent standards for advertising truth and accuracy, delivering meaningful protection to consumers and leveling the playing field for business.
Contact Information
Name: Jennie Rosenberg
Email: jrosenberg@bbbnp.org
Job Title: Media Relations
LexisNexis 2025 Future of Work Report Reveals Active Exploration and Adoption of Generative AI Among Organizations and Professionals
Report shows 80% feel genAI has met or exceeded expectations but insufficient training, lack of trust, and ethical considerations remain barriers to realizing its full value
NEW YORK — February 11, 2025 — LexisNexis® Legal & Professional, a leading global provider of AI-powered analytics and decision tools, today released results from its second annual LexisNexis Future of Work Report, which demonstrates growing maturity and active exploration of generative AI (genAI) adoption. With more than 1,800 responses from professionals globally, the report reveals that 82% of respondents are open to the adoption of genAI technologies and products, with 73% indicating confidence in genAI’s capabilities and expecting a positive impact on day-to-day tasks.
Most notably, the survey reveals that professionals are no longer cautiously experimenting with genAI but are actively utilizing the technology to reap significant benefits in productivity and efficiency. Compared to LexisNexis’ 2024 Future of Work Report, the 2025 survey shows that respondents no longer see genAI as somewhat experimental but as a performance booster, with 80% noting genAI has met or exceeded their expectations. Significantly, 53% of professionals also noted saving one to two hours per day with genAI while 30% saw a three- to four-hour savings, reflecting a clear return on investment for organizations implementing the technology.
“As genAI becomes more integral to business, it’s transforming how we innovate and solve problems. To keep this momentum, we need to strategically implement AI tools that enhance human expertise and shape the future of work,” said Snehit Cherian, Chief Technology Officer of Nexis Solutions, a division of LexisNexis. “By using genAI effectively, we can boost productivity, drive innovation, and ensure long-term success.”
Additional insights and findings show:
- GenAI Reshaping Industries and Automating Tasks: GenAI continues to reshape industries including Professional Services & Consulting, Financial Services, Technology, Healthcare, and Education, with technology-focused industries showing the highest adoption of AI tools, and the financial sector also showing strong engagement. In terms of tasks, the top three areas where genAI excels among professionals are automating routine tasks, data analysis and insights, and creating written content.
- C-Suite Leaders Remain Committed to GenAI and Innovation: C-Suite leaders continue to demonstrate a strong readiness to utilize genAI, with more than half being “extremely” open to these new technologies and 84% being open overall – the highest level among all the career stages – underscoring their ongoing commitment to innovation.
- Generational and Career Synergy Enhances AI Integration: A blend of generational perspectives and career stages reveals that genAI thrives when experience meets technological fluency. Millennials and Gen-X professionals lead the charge in terms of AI integration, using their digital fluency to enhance productivity and foster innovation, while Gen Z’s inherent tech- savviness promises to further accelerate AI adoption.
Trust, Ethical Considerations and Training Required for GenAI Use and Adoption
While organizations are apt to explore and adopt genAI tools, barriers of trust, ethical concerns, and training must be addressed to attain genAI’s full value. 47% of surveyed organizations indicated concerns about data privacy or security and 44% noted a lack of trust in the accuracy of genAI outputs. When it comes to removing these hurdles, a multi-faceted approach is required, including:
- Clear organizational policies that balance security with innovation
- Better integration solutions for existing systems
- Comprehensive training programs
- Structured approaches to evaluating and implementing AI tools
By aligning AI initiatives with business objectives and continuously adapting to advancements, professionals and organizations can realize significant returns on their genAI investments.
For more details or to access the LexisNexis 2025 Future of Work report, please visit http://lexisnexis.com/futureofwork.
About LexisNexis Legal & Professional
LexisNexis® Legal & Professional provides legal, regulatory, and business information and analytics that help customers increase their productivity, improve decision-making, achieve better outcomes, and advance the rule of law around the world. As a digital pioneer, the company was the first to bring legal and business information online with its Lexis® and Nexis® services. LexisNexis Legal & Professional, which serves customers in more than 150 countries with 11,800 employees worldwide, is part of RELX, a global provider of information-based analytics and decision tools for professional and business customers. For more information, please visit https://www.lexisnexis.com.
Contact Information
Leela Bozonelis, Global Product Marketing Director
Nexis Solutions, a Division of LexisNexis
929-383-8781
leela.bozonelis@lexisnexis.com
Jennifer Johnston, Plat4orm PR
208-989-9962
jennifer@plat4orm.com
National Advertising Review Board Finds T-Mobile’s ‘Save 20% Every Month vs. The Other Big Guys’ Claim Supported

New York, NY – February 6, 2025 – A panel of BBB National Programs’ National Advertising Review Board, the body that reviews appeals of decisions made by the National Advertising Division, determined T-Mobile US, Inc.’s claim, “save 20% every month vs. the other big guys,” is supported.
In the underlying National Advertising Division (NAD) case (#7402), Charter Communications, Inc. challenged T-Mobile’s commercial featuring Patrick Mahomes, Snoop Dogg, and influencer Kai Cenat. In the commercial, T-Mobile advertises that “families can save 20% vs. the other big guys” with T-Mobile wireless service.
In its decision, NAD recommended that T-Mobile discontinue or modify the challenged advertising to make clear the companies that are the object of comparison and avoid conveying the message that customers can save 20% compared to Spectrum Mobile in the first year of service.
The National Advertising Review Board (NARB) panel disagreed with the NAD decision and determined that, in the mobile phone category, the “big guys” are Verizon, AT&T, and T-Mobile, based on their significant market shares. The panel determined that consumers would not consider Spectrum Mobile, with its smaller market share, to be in that category.
Regarding the pricing claim, the NARB panel found that T-Mobile’s claim that consumers can, but not necessarily will, save 20% versus Spectrum Mobile is valid. The panel noted the availability of a “savings calculator” on T-Mobile’s website that allows consumers to calculate possible savings.
In its advertiser statement, T-Mobile stated that it “appreciates the panel’s careful consideration of the parties’ arguments” and “remains a strong supporter of the self-regulation process.”
All BBB National Programs case decision summaries can be found in the case decision library. For the full text of NAD, NARB, and CARU decisions, subscribe to the online archive. This press release shall not be used for advertising or promotional purposes.
About BBB National Programs: BBB National Programs, a non-profit organization, is the home of U.S. independent industry self-regulation, currently operating more than a dozen globally recognized programs that have been helping enhance consumer trust in business for more than 50 years. These programs provide third-party accountability and dispute resolution services that address existing and emerging industry issues, create a fairer playing field for businesses, and a better experience for consumers. BBB National Programs continues to evolve its work and grow its impact by providing business guidance and fostering best practices in arenas such as advertising, child-and-teen-directed marketing, data privacy, dispute resolution, automobile warranty, technology, and emerging areas. To learn more, visit bbbprograms.org.
About the National Advertising Review Board (NARB): The National Advertising Review Board (NARB) is the appellate body for BBB National Programs’ advertising self-regulatory programs. NARB’s panel members include 85 distinguished volunteer professionals from the national advertising industry, agencies, and public members, such as academics and former members of the public sector. NARB serves as a layer of independent industry peer review that helps engender trust and compliance in NAD, CARU, and DSSRC matters.
Contact Information
Name: Jennie Rosenberg
Email: jrosenberg@bbbnp.org
Job Title: Media Relations
MICHELIN LAUNCHES E.PRIMACY ALL-SEASON TIRE TO CONTINUE EFFICIENCY LEADERSHIP

- The Michelin Primacy All Season tire delivers up to 13,000 more miles than two leading competitors.1
- Gas, Hybrid or EV, the Michelin e.Primacy All Season tire is up to 25% more efficient than two leading competitors, providing improved fuel economy and increased range.2
- Michelin Primacy All Season tire allows you to drive up to 20 miles further on a charge than leading competitors.2
GREENVILLE, S.C., Feb 3, 2025 – Michelin, a pioneer in efficiency and the most awarded tire brand in the U.S.3, is launching the new e.Primacy All Season tire to meet the evolving demands of drivers who prioritize efficiency.
“As the market continues to change with the development of more hybrids and EVs, Michelin continues to be an industry leader for confident and lasting performance,” said Omer Waysman, vice president of marketing for Michelin North America, Inc.’s business-to-consumer products. “When fuel efficiency and extended range matter, consumers can trust the e.Primacy All Season tire to take them further on every journey.”
As a trusted original equipment tire line, the Michelin e.Primacy All Season tire is up to 25% more efficient than two leading competitor products, providing up to 20 more miles of battery range and saving you a tank of gas annually2,4. By using the GreenPower Compound, the tire is designed to help reduce energy consumption during everyday use and delivers long-lasting mileage.
The e.Primacy All Season tire endured extensive testing and development; even when tested on an EV, the Michelin e.Primacy All Season tire is expected to last up to 13,000 miles longer than two leading competitors, potentially adding an extra year of driving.5
Whether it’s an ICE, Hybrid or EV, Michelin innovations provide a refined driving experience for all drivers by delivering a comfortable and quiet ride.
Consumers can enjoy the benefits of two different Michelin innovations within the e.Primacy All Season tire. Cushion Guard, which is built with a soft, cushioning layer of rubber between the tread and the steel belts to help absorb road impacts and imperfections for a smooth and luxurious ride.
Intentionally quiet, the e.Primacy All Season tire also includes Piano Acoustic Technology, a tread pattern intelligently optimized to help reduce noise for a quiet drive throughout the life of the tire.
To find out which sizes are available today, please visit michelinman.com.
1Based on Federal Highway Administration Average Annual Miles of 13,476 miles per driver (published in 2022) and a treadwear test using tires in size 235/40R19 on 2024 Tesla Model 3, the Michelin e.Primacy All Season tire showed an estimated life (based on calculating the most-worn groove of a rotated set) of 45,764 miles versus the Bridgestone Turanza EV tire at 30,244 miles and the Continental ProContact RX tire at 32,438 miles. Actual on-road results may vary with driving style, temperature, driver selected vehicle settings, and road conditions.
EV tire at 30,244 miles and the Continental ProContact RX tire at 32,438 miles. Actual on-road results may vary with driving style, temperature, driver selected vehicle settings, and road conditions.
2Based on internal test results on ISO 28580 Rolling Resistance Test which included tires in dimension 235/40R19 96W, comparin g Michelin e·Primacy All Season tire (6.46kg/ton) versus Continental ProContact RX T0 tire (7.24kg/ton) and Bridgestone Turanza  EV tire (8.75kg/ton). Actual on-road results may vary with driving style, temperature, driver selected vehicle settings, and road conditions. Additional calculations were conducted on the USTR 781 test data to compute the effect the rolling resistance figures would have on vehicle range for 2024 Tesla Model 3 RWD BEV and a 2024 BMW M3 gas vehicle. Calculations result in a fuel consumption reduction of up to 3.1% for a 2024 BMW M3 or equivalent gain of up to 8.9% in battery range for a 2024 Tesla Model 3 RWD.
EV tire (8.75kg/ton). Actual on-road results may vary with driving style, temperature, driver selected vehicle settings, and road conditions. Additional calculations were conducted on the USTR 781 test data to compute the effect the rolling resistance figures would have on vehicle range for 2024 Tesla Model 3 RWD BEV and a 2024 BMW M3 gas vehicle. Calculations result in a fuel consumption reduction of up to 3.1% for a 2024 BMW M3 or equivalent gain of up to 8.9% in battery range for a 2024 Tesla Model 3 RWD.
3Michelin tires have been ranked the #1 tire brand by industry experts and consumers alike, across major categories and segments. Please visit www.michelinman.com/auto/awards-and-recognition for more details.
4Based on 2022 Federal Highway Administration Report citing an Average Annual Miles per driver of 13,476 miles.
5Based on Federal Highway Administration Average Annual Miles of 13,476 miles per driver (published in 2022) and a treadwear test using tires in size 235/40R19 on 2024 Tesla Model 3, the Michelin e.Primacy All Season tire showed an estimated life (based on calculating the most-worn groove of a rotated set) of 45,764 miles versus the Bridgestone Turanza EV tire at 30,244 miles and the Continental ProContact RX tire at 32,438 miles. Actual on-road results may vary with driving style, temperature, driver selected vehicle settings, and road conditions.
EV tire at 30,244 miles and the Continental ProContact RX tire at 32,438 miles. Actual on-road results may vary with driving style, temperature, driver selected vehicle settings, and road conditions.
About Michelin North America, Inc.
Michelin, the leading mobility company, is working with tires, around tires and beyond tires to enable Motion for Life. Dedicated to enhancing its clients’ mobility and sustainability, Michelin designs and distributes the most suitable tires, services and solutions for its customers’ needs.
Michelin provides digital services, maps and guides to help enrich trips and travels and make them unique experiences. Bringing its expertise to new markets, the company is investing in high- technology materials, 3D printing and hydrogen, to serve a wide variety of industries — from aerospace to biotech. Headquartered in Greenville, South Carolina, Michelin North America has approximately 22,500 employees and operates 34 production facilities in the United States and Canada. (michelinman.com)
For more information contact:
Contact: Christian Fisher
Email: christian.fisher@michelin.com | www.michelinmedia.com
